The document summarizes updates on corneal cross-linking (CXL) technology from Avedro, including:
1) In March 2015, Avedro received a letter from the FDA requesting further information about equivalency between study devices and commercial systems for CXL to treat keratoconus and ectasia.
2) Agreement was reached on additional measurements required to address the FDA's questions. The application was resubmitted in October 2015.
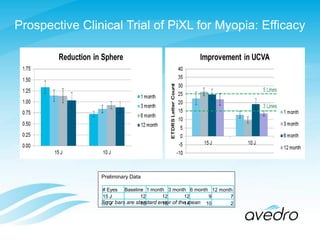
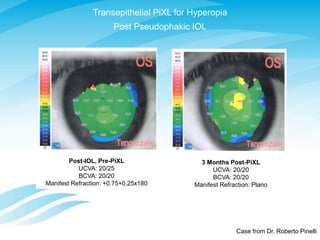
3) Preliminary data is presented on trials of a new CXL technique called PiXL for treating myopia, keratoconus, and post-cataract refractive error, showing safety and efficacy.










![PiXL Clinical Outcomes
• No significant adverse events to date
• Gentle procedure with no tissue removal
• No persistant postoperative glare, haloes, or dry eye
symptoms
“It’s funny how patients who might be good PiXL candidates seem to
be popping out of the woodwork – real field of dreams stuff.”
Dr. Andrew Logan
“Reviewed PiXL today... surgeon and patient ecstatic."
Dr. Paul Hughes
“I am very very very very happy, I just saw my first patient of bilateral
Pixl [for myopia]. She is great and very happy with [her] UCVA”
Dr. Vicente Rodriguez](https://image.slidesharecdn.com/avedro-160204202838/85/Avedro-13-320.jpg)
