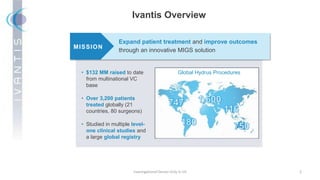
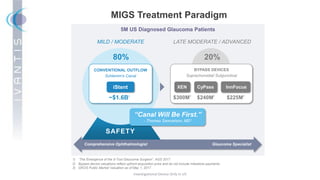
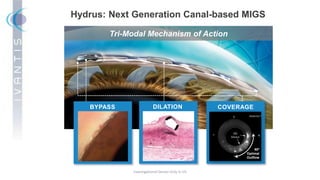
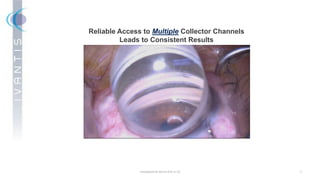
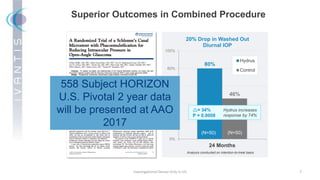
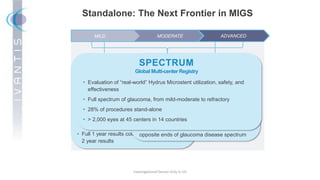
The document discusses the Hydrus Microstent, a minimally invasive glaucoma surgery (MIGS) device that provides a tri-modal mechanism of action to lower intraocular pressure. Over 3,200 patients have been treated globally using the Hydrus Microstent. Clinical studies and a large global registry have shown the Hydrus Microstent to provide superior outcomes compared to other MIGS devices, with a 20% reduction in diurnal intraocular pressure and increased treatment response rates. The Hydrus Microstent expands treatment opportunities for both standalone glaucoma procedures and combined cataract/glaucoma surgeries.