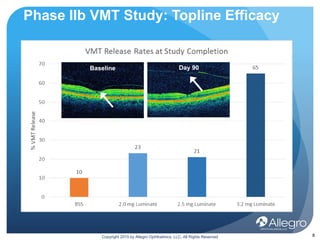
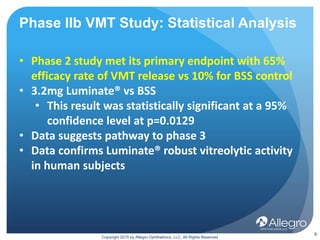
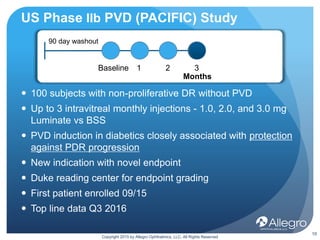
Luminate is a drug being developed by Allegro Ophthalmics to treat retinal diseases. It works through a novel anti-integrin mechanism of action compared to existing anti-VEGF drugs. Phase 2 studies showed Luminate had a 65% efficacy rate in resolving vitreomacular traction, a robust safety profile, and durability of at least 3 months in treating diabetic macular edema and wet age-related macular degeneration. Ongoing phase 2b studies are further evaluating Luminate's potential to treat diabetic macular edema, proliferative vitreoretinopathy, and as an alternative to anti-VEGF drugs with longer duration of effect.