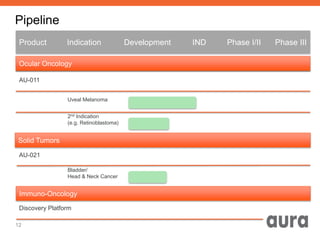
This corporate presentation outlines Aura Biosciences' novel targeted therapy approach using viral-like particles to treat cancer. Their lead product, AU-011, is being developed for the treatment of ocular melanoma, an orphan disease with no approved therapies. Preclinical data shows AU-011 effectively targets and kills tumor cells through a unique mechanism of action. Aura plans to initiate clinical trials in Q1-2/2016 to obtain proof of concept data and accelerate approval. If approved, AU-011 could be the first FDA-approved treatment for the primary tumor in ocular melanoma patients.