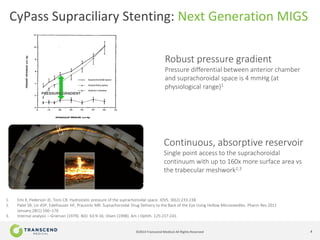
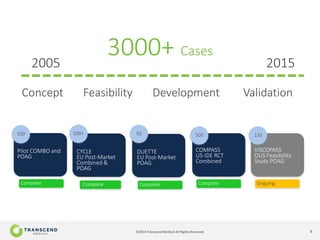
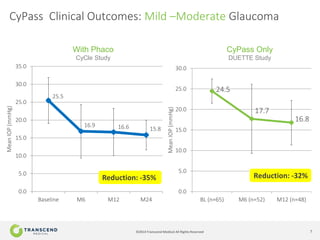
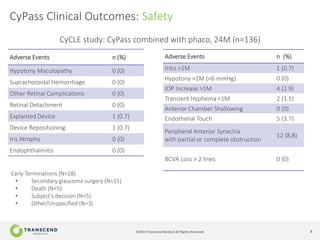
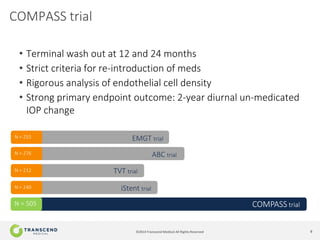
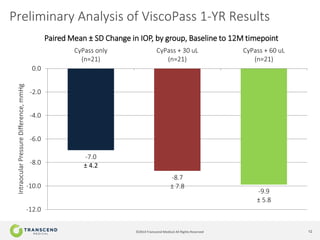
The document discusses CyPass, a novel micro-invasive glaucoma surgery (MIGS) device that uses a supraciliary stent to enhance aqueous outflow through the suprachoroidal space. It provides data from clinical studies showing that CyPass lowers intraocular pressure by over 30% with a favorable safety profile. A new version called CyPass Vx is also mentioned as being developed to treat a broader range of glaucoma severity.