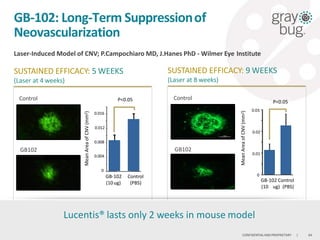
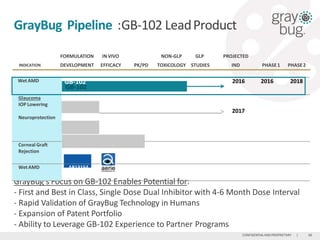
GrayBug is developing an injectable, long-acting ocular drug delivery technology called GB-102 for treating wet age-related macular degeneration. GB-102 is a dual anti-VEGF/anti-PDGF therapeutic that enables injections every 4-6 months, compared to monthly injections currently required. GrayBug has worldwide rights to the technology from Johns Hopkins University and aims to file an IND for GB-102 in wet AMD in 2016. The company is also exploring applications of its technology for glaucoma and other ocular diseases, with the goal of developing long-acting drugs that improve compliance over daily eye drops.