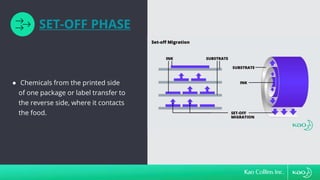
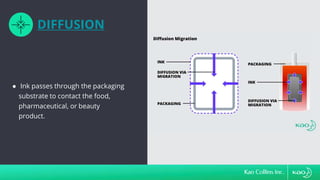
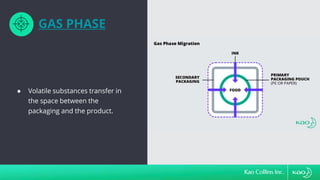
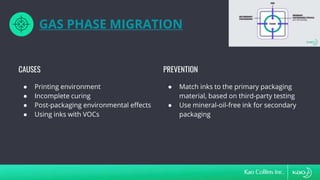
The document discusses ink migration in food packaging, highlighting risks such as consumer health issues, flavor changes, and regulatory violations due to improper printing processes. It outlines types of migration (set-off, diffusion, and gas phase) and prevention measures like using tested inks and proper curing practices. Additionally, it emphasizes good manufacturing practices (GMPs) for quality control in packaging production to ensure compliance and minimize contamination risks.