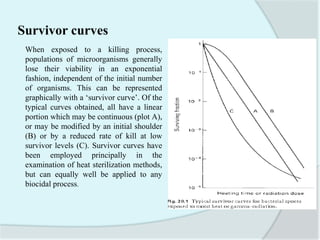
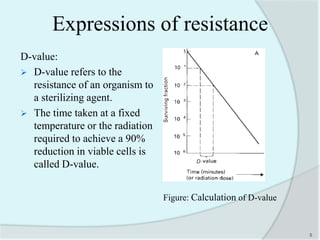
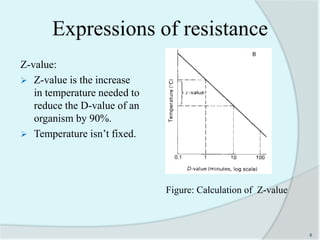
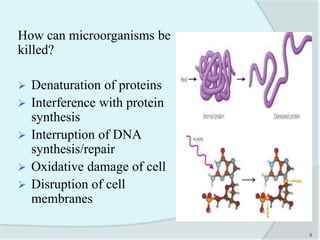
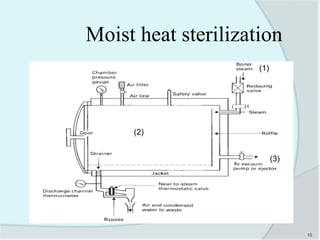
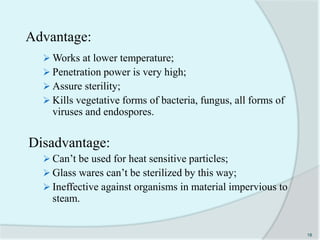
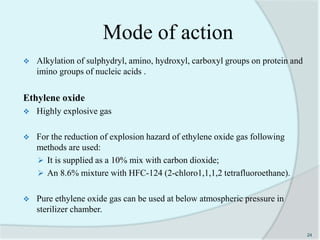
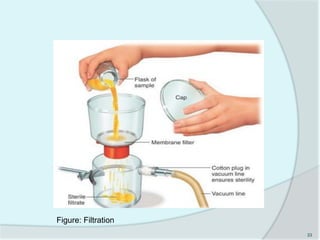
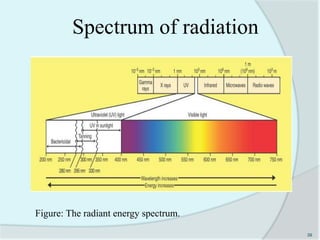
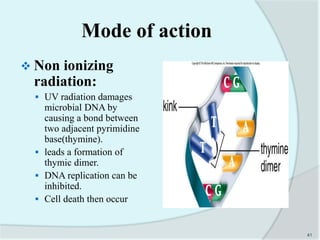
The document details the practice of sterilization in pharmaceuticals, highlighting methods such as physical (moist heat, dry heat, radiation, and filtration) and chemical (gaseous sterilization). It emphasizes the importance of eliminating microorganisms to ensure safety and sterility, and introduces key concepts like D-value, Z-value, and various sterility assurance measures. The document also discusses the advantages and disadvantages of each sterilization method and their application in sterilizing different medical and pharmaceutical materials.