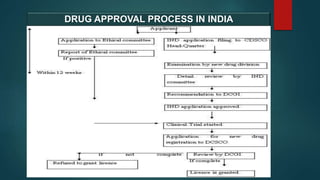
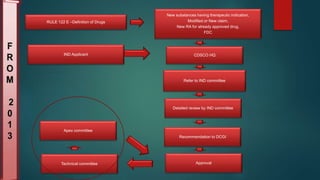
The document outlines the regulatory framework for drug approval and clinical trials in India, governed primarily by the Drugs and Cosmetics Act of 1940 and associated rules. It details the processes required for submitting applications for Investigational New Drugs (INDs) and New Drug Applications (NDAs), including necessary documentation and timelines for approval. Additionally, it highlights the roles of various committees and the legal hierarchy that oversees the compliance and ethical guidelines in drug development.