LEDs emit light when an electric current passes through a semiconductor material. They are used widely in displays, lighting, and other applications due to their low power use, long lifespan, and fast switching. LEDs work via electroluminescence - when electric current passes through the diode, electrons recombine with holes and release photons. The color of light emitted depends on the semiconductor material used. Solar energy is the energy from the sun that can be converted to heat or electricity via solar panels. It is a renewable resource but has disadvantages like lower output during winter and cloudy weather.




























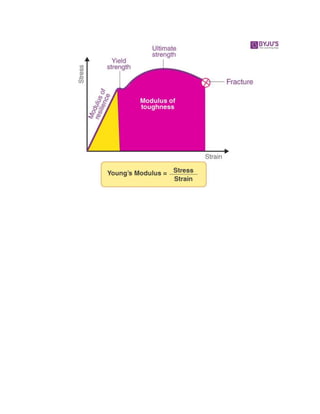
![Young’s Modulus
Give examples of dimensionless quantities.
Following are the examples of dimensionless quantities:
Poison’s ratio
Strain
Give an example of a material with the highest elasticity.
Steel is an example of a material with the highest elasticity.
What is ductility?
Ductility is defined as the property of a material by which the material is drawn to a smaller
section by applying tensile stress.
What is the dimensional formula of Young’s modulus?
The dimensional formula of Young’s modulus is [ML-1
T-2
].
What is the SI unit of Young’s modulus?
Pascal is the SI unit of Young’s modulus.
What is Young’s Modulus?
The mechanical property of a material to withstand the compression or the
elongation with respect to its length.](https://image.slidesharecdn.com/importantquestionsforviva-converted1-converted-220509081940-ecfde5e3/85/Important-Questions-For-Viva-converted-1-converted-pptx-38-320.jpg)