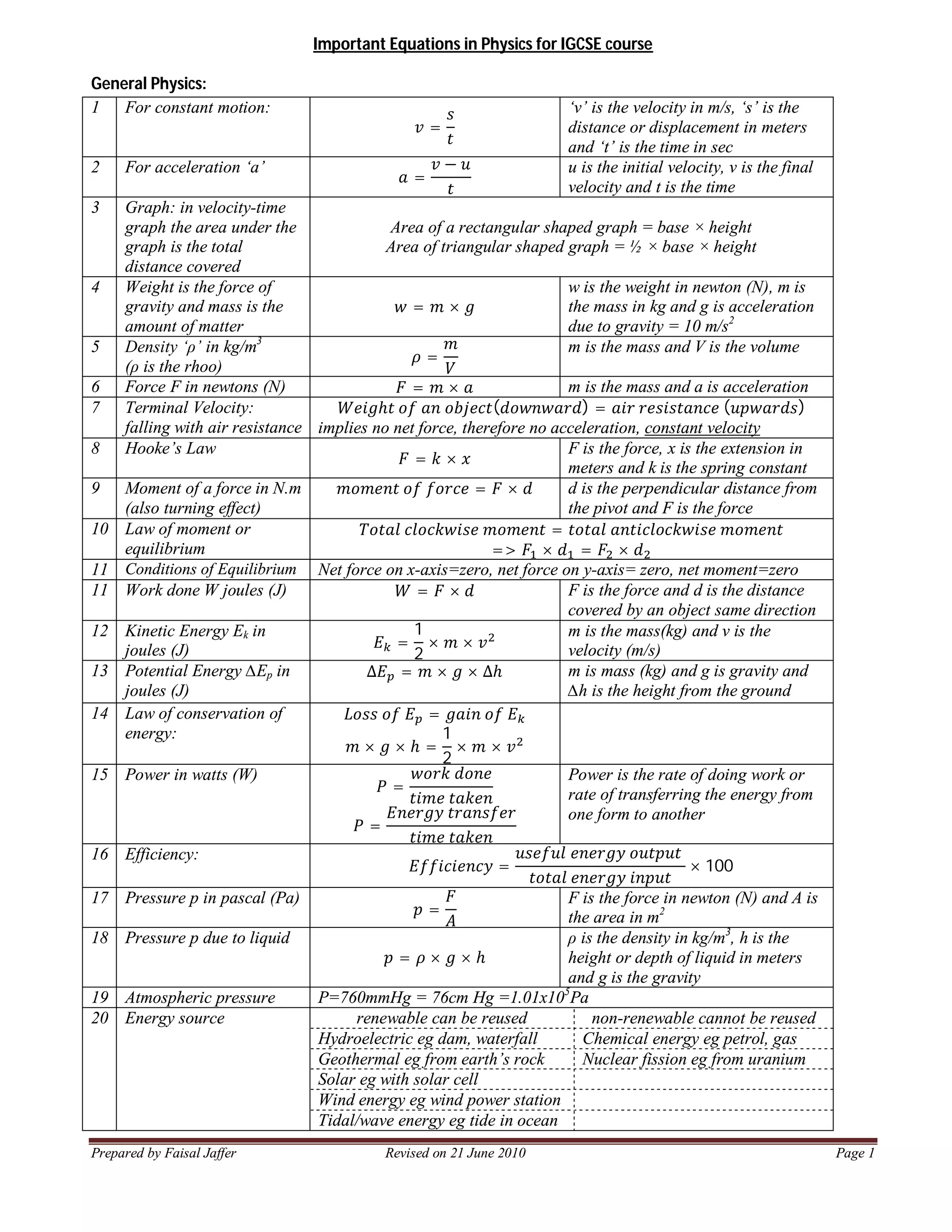
This document provides important equations in physics for the IGCSE course. It covers equations for general physics, thermal physics, waves, light and sound, and electricity and magnetism. Some key equations included are:
1) Velocity = Distance/Time for constant motion.
2) Acceleration = Change in Velocity/Time.
3) Hooke's Law: Force = Spring Constant x Extension.
4) Boyle's Law: Pressure x Volume = Constant for gases.
5) Wave Equation: Velocity = Wavelength x Frequency.
6) Refractive Index = Speed of Light in Medium / Speed of Light in Vacuum.
7) Ohm's Law: Voltage = Current x Resistance.