This study investigated the inhibition of copper corrosion in nitric acid solution by 3-arylazotriazole compounds (AT). Potentiodynamic polarization and Tafel methods showed that AT compounds were good inhibitors. The inhibition effect is likely due to adsorption of AT molecules and formation of Cu(II)-AT complexes on the electrode surface. Higher AT concentrations achieved over 95% inhibition efficiency. Temperature increased corrosion rates by enhancing desorption and surface reactions. UV-Vis spectroscopy and cyclic voltammetry supported the formation of Cu(II)-AT complexes in solution.
![Monatshefte fiir Chemie 126:1087-1095 (1995)
Monatshefte///r Chem/e
Chemical Monthig
© Springer-Yerlag 1995
Printed in Austria
Inhibition of Copper Corrosion by Arylazotriazoles
in Nitric Acid Solution
L. H. Madkour, M. A. Elmorsi, and M. M. Ghoneim
Chemistry Department, Faculty of Science, Tanta University, Tanta, Egypt
Summary. A study has been made to investigate the effect of some azoheterocyclic dyes of the type
3-arylazo 1,2,4-triazole (AT) on the corrosion of copper exposed to 0.5M nitric acid solution at
different temperatures and at different AT concentrations. Using potentiodynamic polarization and
Tafel electrochemical methods, it can be shown that AT compounds are good inhibitors of copper
corrosion in HNO 3 solution. The kinetic and thermodynamic parameters of the inhibited system were
determined. The high inhibition efficiency of these compounds may be due to the adsorption of the
additive itself and/or the adsorption of the formed Cu(II)-AT complexes at the polarized electrode
interface. Cathodic polarization measurements showed that AT dyes are predominantly cationic
inhibitors.
Keywords. Acid medium; Arylazotriazoles; Inhibition; Copper.
Hemmung der Korrosion yon Kupfer in salpetersaurer Liisung durch Arylazotriazole
Zusammenfassung. Der Effekt einiger azoheterocyclischer Farbstoffe des Typs 3-Arylazo-1,2,4-triazol
(AT) auf die Korrosion yon Kupfer in 0.5M Salpeters/iure bei verschiedenen Temperaturen und
unterschiedlichen AT-Konzentrationen wurde untersucht. Potentiodynamische Polarisation und
elektrochemische Methoden nach Tafel zeigen, dab Verbindungen vom Typ AT gute Korrosions-inhibitoren
fiir Kupfer gegeniiber HNO 3 sind. Die kinetischen und thermodynamischen Parameter
des inhibierten Systems wurden bestimmt. Die hohe inhibitorische Wirksamkeit der untersuchten
Verbindungen kann auf die Adsorption des Additivs selbst oder auf jene des Cu(II)-A T-Komplexes
an der polarisierten Elektrodenoberfl/iche zuriickzufiihren sein. Kathodenpolarisationsmessungen
zeigen, dab A T-Farbstoffe vorwiegend kationische Inhibitoren sind.
Introduction
Copper is widely used in various industrial and constructional operations.
Consequently, the study of its corrosion inhibition is of great importance today.
Many studies have been devoted to the inhibition of copper corrosion in neutral
and acidic media by benzotriazole or tolytriazole compounds [1-5], but there are
relatively few investigations on arylazotriazoles. It has been suggested that the
benzotriazole compounds act by forming an organometallic complex molecular
layer at the electrode surface. In this work, the authors present a study of the
inhibiting effect of some 3-arylazo 1,2,4-triazoles on the corrosion of copper in
aerated 0.5 M HNO 3 solution.](https://image.slidesharecdn.com/1995complete-141025132417-conversion-gate01/75/1995-complete-1-2048.jpg)
![1088 L.H. Madkour et al.
Results and Discussion
Inhibitor's effect on corrosion
Potentiodynamic measurements were performed in the presence of different
concentrations of arylazotriazole dyes added to 0.5 M HNO 3 solutions at 295 K.
The results are shown in Table 1 and Fig. 1. The three arylazotriazole dyes D 1, D 2,
and D 3 attain an optimum inhibition of > 95% at a concentration of 10 .4 M. The
high inhibition efficiency of the applied dyes towards copper can be explained as
follows. As the dye molecule approaches the electrode surface, the electric field of
the double layer increases the polarization of the molecule and induces additional
charges on both the N and O atoms, thus enhancing the adsorption of the dye
molecule. In the case of D 1 and D 2, the formation of Cu(II)-dye complexes in solution
can be assumed which consequently are adsorbed at the electrode surface. The
formation of these complexes between Cu 2÷ and D1 or D2 and the mechanism of
their chelation was explained by a proton displacement from the phenolic OH group
by the Cu 2 + ion [6]. Thus, the bonding of the Cu 2 ÷ ion to the dye molecule takes
place through a covalent linkage with the oxygen of the phenolic group, whereas
the N=N group contributes to a coordination bond as follows:
N--TN:N~O /OH2
L _N ~ Cu--H20 -Y" 1".o
H H20
Cu(ll)-D1 complex
HI
f NN Me
N- ~1 N%
H20 N~,~ )) o_ o _o
t OH2
~ / %N--~-- N
Me I~.
"~N /
I
H
Cu(II)-D2 complex (1:2; at high concentration of D2)
H2Ox." ?H2
/______~O-- (iu NO2
~( ))-~ N. OH2
~"/ ~N~N
Me 1~.
--.N j
I
H
Cu(II)-D 2 complex (1:1; at low concentration of £)2)](https://image.slidesharecdn.com/1995complete-141025132417-conversion-gate01/85/1995-complete-2-320.jpg)
![Inhibition of Copper Corrosion 1089
• I.gO~ . Free acid /tg
xxxxx 1xlt~'M O 1 //;J /
...... 1,1o4M o2 I,': /
..... ,,,o,Mo3 /# /
+20C /'g
j
+I00 ~~, ~ "'~
LU
-10C -.... "-....... ,% ,,
-20C ""-... ""-%
%.,.
I ] I i , I
103 10 z 105 106 10 7 108 109
i, n ,4 cm -2
Fig. 1. Potentiodynamic polarization curves of Cu in 0.5 M HNO 3 alone and containing 10-4M
arylazotriazoles (Dx, D 2, and D3) at 295 K
t~
(b
25
1.5
0.5-
2OO
f~
400 600
wove lengfh ~rim
Fig. 2. Absorption spectra of Cu in 0.5 M HNO 3 con-taining
l0 -4 M D2; : no corrosion; .:
corrosion at 313 K; xxxx: corrosion at 333 K
+0.35
+0.15
z,, -O.O5
- 0..25
-0.~5
÷0.25
,-, //
",, .,">. i '
" ",~,i"
, _t , I , 1
*0.05 -0.15 -q35
E(v vs. ,4g*)
Fig. 3. Cyclic voltammograms of Cu in 0.5 M
, HNO3 containing I0-4M D 3 ; - - : no
-0.50 corrosion; ; corrosion at 295K;
..... : corrosion at 333 K](https://image.slidesharecdn.com/1995complete-141025132417-conversion-gate01/85/1995-complete-3-320.jpg)

![Inhibition of Copper Corrosion 1091
Cu 2 + complexes readily forms with the title dyes D1 and D2; it has been proved that
the ratio of the metal ion to the ligand either is 1:1 or 1:2. The structure of the
resulted complexes was substantiated by their analytical data, by cyclic voltammetry,
and by spectral analysis (mainly ultraviolet spectra) as shown in Figs. 2 and 3.
The inhibition effect of the three studied arylazotriazole compounds D 1, D2, and
D 3 are likely due to the interaction of the adsorbed dye molecules with the Cu 2 +
ions at the electrode surface formed during the polarization at the interface via the
triazole ring forming a chemisorbed complex [7]. From Table 1, Ecorr becomes more
negative at the addition of the inhibitors which indicates that these tested dyes are
predominantly effective in the cathodic region.
Tqfel constants for Cu corrosion
The cathodic Tafel constant (CTC) in the free nitric acid solution corresponds to
oxygen reduction, whereas the higher anodic Tafel constant (ATC) values (Table 1)
mainly correspond to the dissolution reactions [8] as fellows:
Cu~Cu ++e- and Cu~Cu 2++2e-
At different concentrations of the tested 3-arylazo 1,2,4-triazoles, it was found that
CTC varied from 66-170 inV. dec-l, whereas ATC changed from 73-141 mV-dec-1.
These higher Tafel constant values support the probability of the presence of a
complicated surface processes involving Cu ions and A T molecules. Moreover, Tafel
slopes of about 90 mV.dec-1 can be attributed to a surface kinetic process rather
than a diffusion controlled process [91.
Temperature effect on corrosion
Ioorr and Rcorr increase with increasing temperature as shown in Table 2. This is due
to the thermal activation of the surface polarization of the following reactions:
Cu~Cu ++e- and Cu~Cu 2 ++2e-
The increase of Rco~r is due to the absence of protective oxide layers at the Cu surface
in nitrate medium. Thus, the increase of temperature enhances both the copper
corrosion and the additive desorption processes without leading to Cu(II)-AT
complex formation. For O2, the inhibiting efficiency value decreased to 56~o at
313 K, then increased again to 83~ at 343 K. It was also found that the colour of the
electrolyte solution containing D 2 changed from yellow to reddish at 313 K and
became violet at higher temperatures. The phenomenon of the colour changes for the
inhibitor D 2 can be explained as follows:
(1) The transformation of the tetrahedral Cu(II)-D2 complex into the octahedral form
by coordination of two solvent molecules leads to the six coordinate complex.
(2) The thermochromic property of Cu-D2 chelates results from a changed
resonance by chelate ring formation; this is due to the intramolecular H-bond
and/or metal chelation.
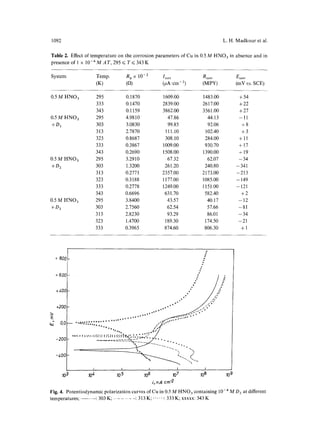
The effect of temperature on the potentiodynamic polarization plot of Cu
exposed to 0.5M HNO3 acid and in the presence of inhibitor D z is plotted in Fig. 4.](https://image.slidesharecdn.com/1995complete-141025132417-conversion-gate01/85/1995-complete-5-320.jpg)
![Inhibition of Copper Corrosion 1093
8
6
2
2
"*, .... D1
'~ - - D2 ,k. I ..... D3
~__L(K ) '~
I"
Fig. 5. logk vs. 1/T plot of Cu in 0.5M HNO3
x I0" containing 10 4M arytazotriazole dyes; xxxxx:
D1; : D2; ..... : D3
It is clear that the passive layer region decreases as the temperature increases. Thus,
D 2 may act as an anodic inhibitor rather than a cathodic one, whereas Ecorr becomes
more positive as the temperature increases (Table 2 and Fig. 4).
The UV spectroscopic investigations and cyclic voltammetric analyses of the
electrolyte solutions containing D 2 and/or D 3 at different temperatures are plotted
in Figs. 2 and 3, respectively. Before the corrosion process, a strong absorption peak
at 303 nm was observed, corresponding to the azo group. During the corrosion
processes, another additional broad absorption band was recorded at 542nm,
corresponding to the formation of the Cu(II)-D2 complex in solution (Fig. 2).
Cyclic voltammetric analysis of the electrolyte solution containing 10-4M D 3
(Fig. 3) revealed one redox peak before the corrosion process. During corrosion at
different temperatures, another additional redox shoulder and redox peak were
observed corresponding to the stepwise reduction of the free Cu 2 + ions present in
solution.
Both UV spectral and cyclic voltammetric analyses indicate that the increase of
temperature enhances both the Cu corrosion and the dye desorption processes.
Kinetic and thermodynamic parameters
The activation energy (AE #) for the copper corrosion in 0.5 M HNO 3 containing
10--4M of different inhibitors (D1, D2, and D3) was calculated from Eyring's
equation k = KTexp [10]. It was found that AE # increases in the order
D 2 > D 3 > D 1. An Arrhenius plot indicating the variation of log k or logRcorr with
the temperature is shown in Fig. 5. The higher entropy value (AS #) in the case of D 2
compared to the values ofD 1 and D 3 as shown in Table i indicates a slower reaction
[11].
Conclusion
The general corrosion of Cu exposed to 0.5 M HNO 3 solution containing arylazo
1,2,4-triazole (A T) dyes can be summarized as follows:
(1) A higher corrosion inhibition efficiency was observed for Cu exposed to 0.5 M
HNO 3 solution containing A T compounds due to the formation of Cu(II)-A T
complexes at the polarized electrode surface. The adsorption process is
endothermic in nature and causes a reasonable of corrosion inhibition at high
temperatures.](https://image.slidesharecdn.com/1995complete-141025132417-conversion-gate01/85/1995-complete-7-320.jpg)
![1094 L.H. Madkour et al.
(2)
(3)
The Cu corrosion inhibition process due to the formation of Cu(II)-AT
complexes is entropy controlled rather than activation energy controlled, since
the activation energy values are relatively high (Table 1).
The higher cathodic Tafel constant value in free acid (0.5 M HNO3) is attributed
to the oxygen reduction, whereas the anodic Tafel constant value represents the
Cu dissolution. After addition of the AT compounds, the cathodic and anodic
reactions mainly involve A T molecules, i.e. a surface kinetic controlled process
takes place.
Experimental
The copper metal (99.99~ Copper Egyptian Company) was used as foils with a surface area of
1-1.3 cm 2, containing 0.001~o Pb as impurity. These foils were sealed in glass tubes using epoxy resin.
The copper electrodes were mechanically polished and rinsed in an ultrasonic bath containing distilled
water before each experiment.
A stock solution of HNO3 (0.5 M) was prepared. Potentiodynamic measurements were maintained
using a computerized corrosion measurement console 350 A-PARC (from EG & G) connected to a
K-47 corrosion cell containing twin high density, non-permeable graphite counter electrodes and a
saturated calomel electrode (SCE) fitted in a bridge tube incorporating an ultra-low leakage Vycor
frit. The cell was immersed in a thermostatted water bath. In the Tafel condition, a controlled potential
scan was applied to the copper electrode starting at E .... and extending in either the anodic or the
cathodic direction for a few hundred millivolts. An extension of the linear region defines i .... at the
intersection with Eoor,. The corrosion rate R .... in milli-inches per year (MPY) can be obtained from
the following equation:
R .... =0.13i .... EW/d
Here, i .... is the corrosion current density (#A-cm-2), EW is the equivalent weight of copper, and d is
its density. Further corrosion measurement details have been given elsewhere [12-14].
The UV/Vis spectra were recorded with a UV-160 A Shimadzu spectrophotometer. The cyclic
voltammograms were recorded using the polarographic analyzer Model 264A and the electrode
assembly (303A) of 2.6 x 10-2 cm 2 area with a sweep rate of 20 mV" sec-1.
The synthesis of the investigated azo-heterocyclic dyes of the type 3-arylazo 1,2,4 triazole
compounds was accomplished directly via the coupling reaction of the diazonim salt of 3-amino
1,2,4-triazole with different types of phenolic compounds.
The investigated arylazatriazole dyes were:
GHO
N--~1~N:N~~ OH
~.N/N
I
H
3-( 3-formyl-4-hydroxg- l-phenylazo)- l ,2,4-triazole ( D1)
HO N 7-N=N- LN N
I
H
3-( 2-hydroxy-5-methyl- l -phenylazo )- l ,2, 4-triazole (D2)](https://image.slidesharecdn.com/1995complete-141025132417-conversion-gate01/85/1995-complete-8-320.jpg)
![Inhibition of Copper Corrosion 1095
N ,[--N:N-~OH
II i~
L..N/N
I
H
3-(4-hydroxy-l-phenylazo)-l,2,4-triazole (/:)3)
References
[1] Mansfeld F, Smith T, Parry EP (1971) corrosion 27:289
[2] Poling GW (1970) Corros Sci 10:359
[3] Fox PG, Lewis G, Bonden PJ (1979) Corros Sci 19:457
[4] Hollander O, May PC (1985) Corros Nace 41:39
[5] Zichuman G, Tong R, Notoya T (1991) B Electrochem 7:60
[6] Gaber M, Hassanein M, Ahmed HA (1986) Indian J Textile Res ll: 48
[7] Chadwick D, Hashemi T (1978) Corros Sci 18:39
[8] Faita G, Fiori G, Salvadore D (1975) Corros Sci 15:383
[9] Dahar HP, White RE, Burnell G, Cornwell LR, Griffin RB, Darby R (1985) Corros Nace 41:317
[10] Parhetier; Souchay "Chemical Kinetics". Elsevier, New York (1967): 155
[11] Taqui Khan MM, Shukla RS (1991) Polyhedron 10:2711
[123 Elmorsi MA, Mabrouk EM, Issa RM, Ghoneim MM (1987) Surface Coatings Technol 30:277
[13] Elmorsi MA, Ghoneim MM, Issa FM, Mabrouk EM (1987) Surface Coatings Technol 31:389
[14] Elmorsi MA, El-Sheikh MY, Bastawessy AM, Ghoneim MM (1991) B Electrochem 7:158
Received January 10, I995. Accepted (revised) April 24, 1995](https://image.slidesharecdn.com/1995complete-141025132417-conversion-gate01/85/1995-complete-9-320.jpg)