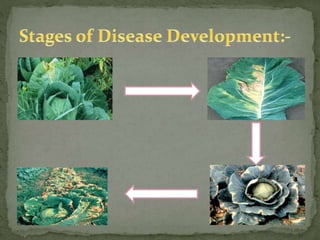
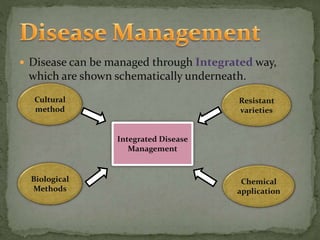
The document discusses the Brassicaceae family, which includes important vegetable crops like cabbage, broccoli, and cauliflower. It covers the taxonomy, economic importance, and cultivation of crops in the family. It also describes several diseases that affect Brassicaceae crops, their symptoms, causal agents, and methods for integrated disease management, including cultural practices, use of resistant varieties, biological control, and chemical applications.



















![ Calcium nitrate [Ca(NO3)2] is effective as a nitrogen source
because of its alkalinizing properties (Dobson et al., 1983).
Another N source Calcium Cyanamide “Perlka” – a very popular
fertilizer in Europe manufactured by Syngenta contain 50%
calcium oxide, 19.8 % nitrogen, 1.5 % magnesium oxide.
Calcium cynamide have both herbicidal and fungicidal
properties and has shown good potential for clubroot control
(Klasse, 1996) provided that the application be made 1 week
before planting.
Moderate to high-risk sites, additional protection in the form of
and boron and/or a protectant fungicide such as fluazinam can
prevent the disease .
Chemical control is difficult because it is a pathogen of soil.](https://image.slidesharecdn.com/idmpractisesonbrassicavegetables-160826120912/85/Idm-practises-on-brassica-vegetables-21-320.jpg)