The document discusses normal oocyte morphology and the process of oogenesis in female mammals, detailing the cellular composition of follicles and stages of meiotic development. It emphasizes the impact of oocyte quality on fertilization and embryo development, highlighting various morphological and cytoplasmic features that can serve as predictors of developmental competence. Key findings include the significance of cumulus-corona cells, nuclear maturity assessment, and cytoplasmic inclusions in determining oocyte quality.

![abnormal oocyte morphology
Mounting evidence that oocyte quality profoundly
affects fertilisation and subsequent embryo
development drives the continued search for
reliable predictors of oocyte developmental
competence. (1)
These criteria are specifically classified as
morphological and cellular/molecular predictors. (1)
. Oocyte quality is not only influenced by the nuclear
and mitochondrial genome, but also by the
microenvironment provided by the ovary and the
pre-ovulatory follicle that influences transcription
and translation, and as a consequence, cytoplasmic
maturity (2) .
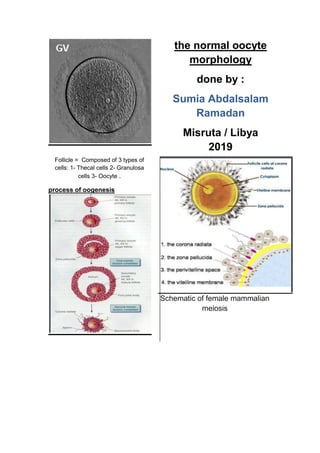
Morphological oocyte assessment is based on the
aspects of : 1- Cumulus-corona cells 2- if
denudation is performed 3- Oocyte morphology : a
rapid evaluation using an inverted microscopic is
also performed after denudation; the cytoplasm, the
perivitelline space, the first polar body, the zona
pellucida. provides very superficial and approximate
information about; the stage of development
[germinal vesicle, metaphase I (MI) or MII phase]
the quality [degenerative signs in the cytoplasm,
polar body (PB) or zona pellucida] Oocyte quality
assessment (2)
A. Cumulus-enclosed oocytes Oocyte
morphological assessment in the laboratory is
first based on the presentation of the cumulus –
corona cells. (2) . During follicular antrum
formation, granulosa cells (GCs) differentiate
into mural GCs, lining the follicular wall, and
CCs, surrounding the oocyte. Within the
cumulus mass, CCs in close contact with the
oocyte (corona cells) develop cytoplasmic
projections which cross the ZP and form gap
junctions with the oolemma. This organized
structure is called the cumulus –oocyte complex
(COC (2); For mature oocytes, the cumulus–
corona mass should appear as an expanded
and mucified layer, due to active secretion of
hyaluronic acid. This extracellular matrix
molecule interposes between the cumulus cells
(CCs), eparating them and conferring to the
cumulus –corona mass a fluffy ‘cloud-like’
appearance. (2) Immature COC: Dense compact
cumulus if present. Adherent compact layer of
corona cells. Ooplasm if visible with the presence of
germinal vesicle. Compact and non-aggregated
membrana granulosa cells](https://image.slidesharecdn.com/normalandabnormaloocytemorphology-190424064320/85/Normal-and-abnormal-oocyte-morphology-3-320.jpg)