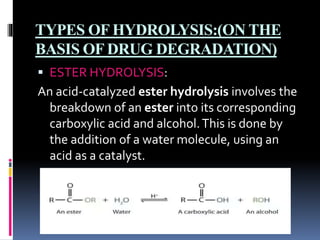
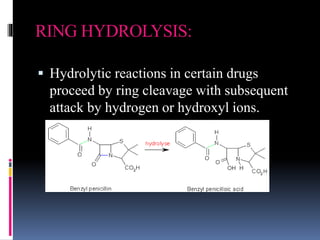
Hydrolysis is the chemical breakdown of a compound due to reaction with water. There are two main types of hydrolysis: ionic and molecular. Ionic hydrolysis occurs when salts of weak acids and bases interact with water, while molecular hydrolysis is a slower, irreversible process catalyzed by hydrogen or hydroxyl ions. The document further discusses three types of hydrolysis based on drug degradation: ester hydrolysis, amide hydrolysis, and ring hydrolysis. Ester hydrolysis involves the breakdown of esters into carboxylic acids and alcohols using an acid catalyst. Amide hydrolysis occurs when amides are hydrolyzed in dilute acid. Ring hydrolysis proceeds by ring cleavage and subsequent attack