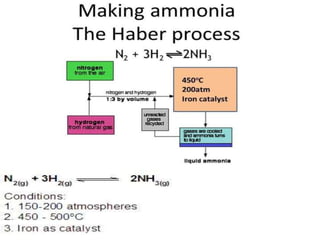
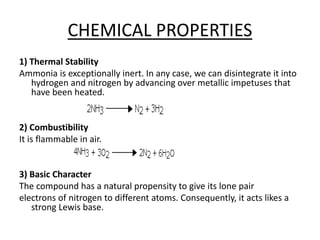
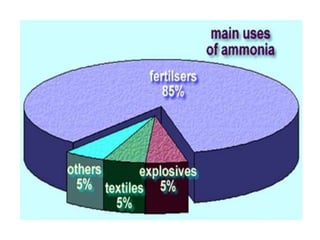
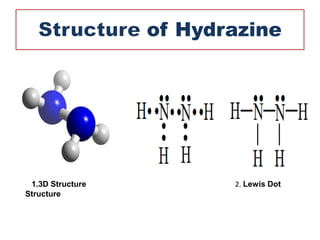
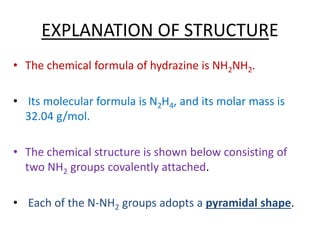
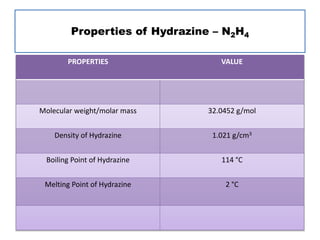
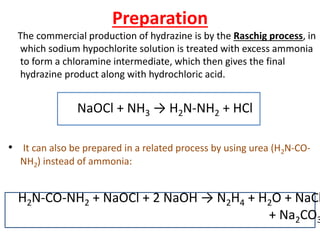
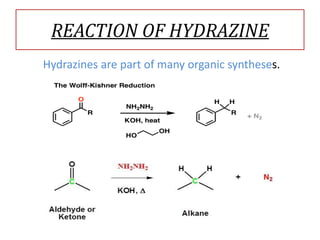
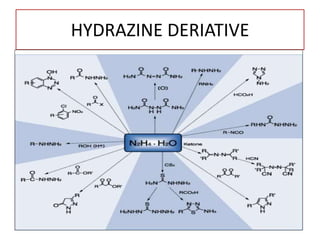
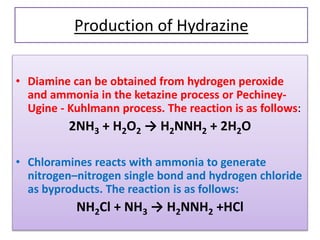
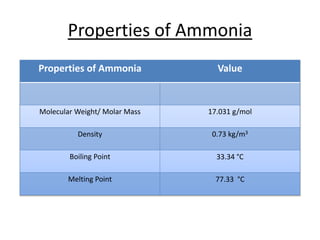
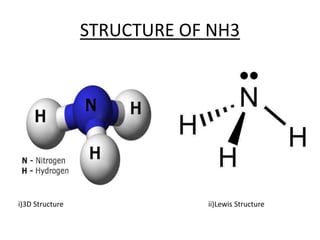
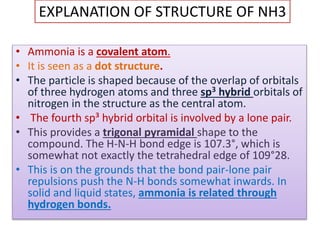
This document discusses hydrides of nitrogen, including ammonia and hydrazine. It provides details on their structures, properties, preparation methods, and uses. Ammonia is a colorless gas that is lighter than air. It is prepared through reactions involving ammonium chloride or metal nitrides. Ammonia is used as a fertilizer and cleaning product. Hydrazine is a reducing agent that exists as a colorless liquid. It is prepared through reactions of sodium hypochlorite with ammonia or urea. Both compounds are discussed in terms of their molecular structures and chemical properties.




![PREPARATION
1)From Ammonium Chloride
We can generate ammonia gas in the research centre by slowly heating ammonium
chloride (NH4Cl) and slaked lime [Ca(OH)2].
REACTION:
2)By the Hydrolysis of Metal Nitrides
Hydrolyzing metal nitrides like magnesium and aluminium nitrides, with
water or alkalis, can likewise deliver ammonia gas.
REACTION:](https://image.slidesharecdn.com/gechemistryassignment-200513161324/85/HYDRIDES-OF-NITROGEN-9-320.jpg)