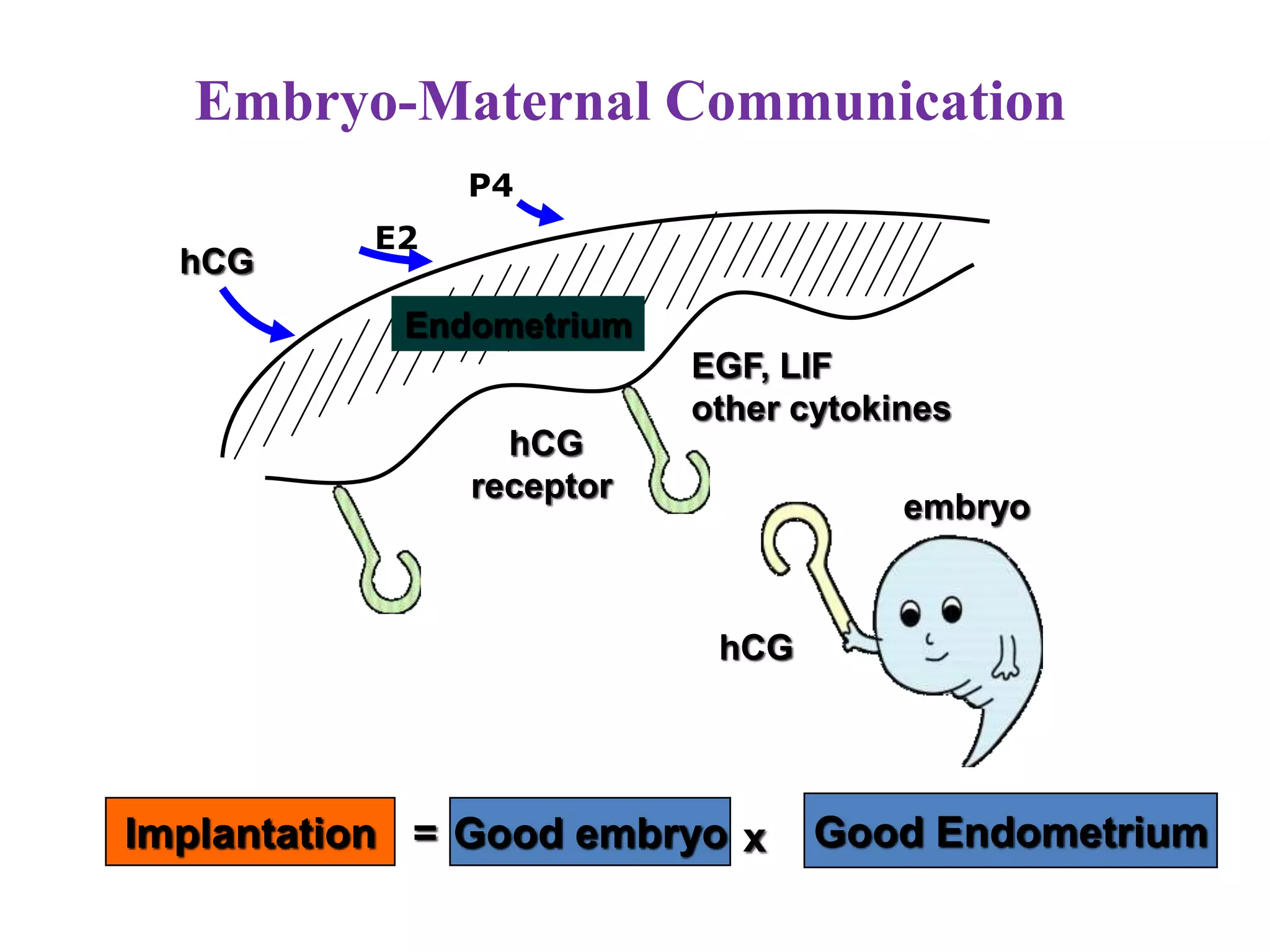
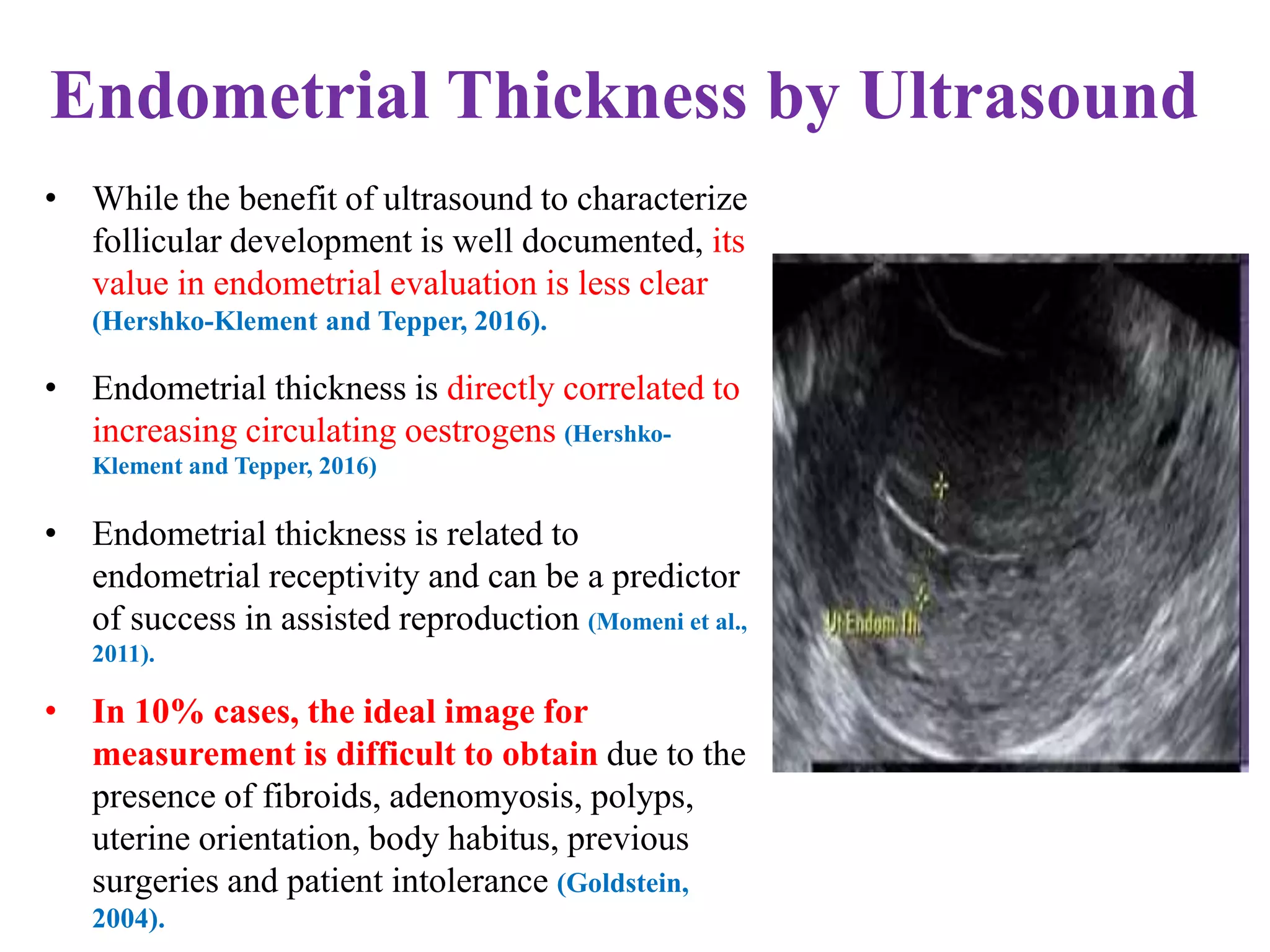
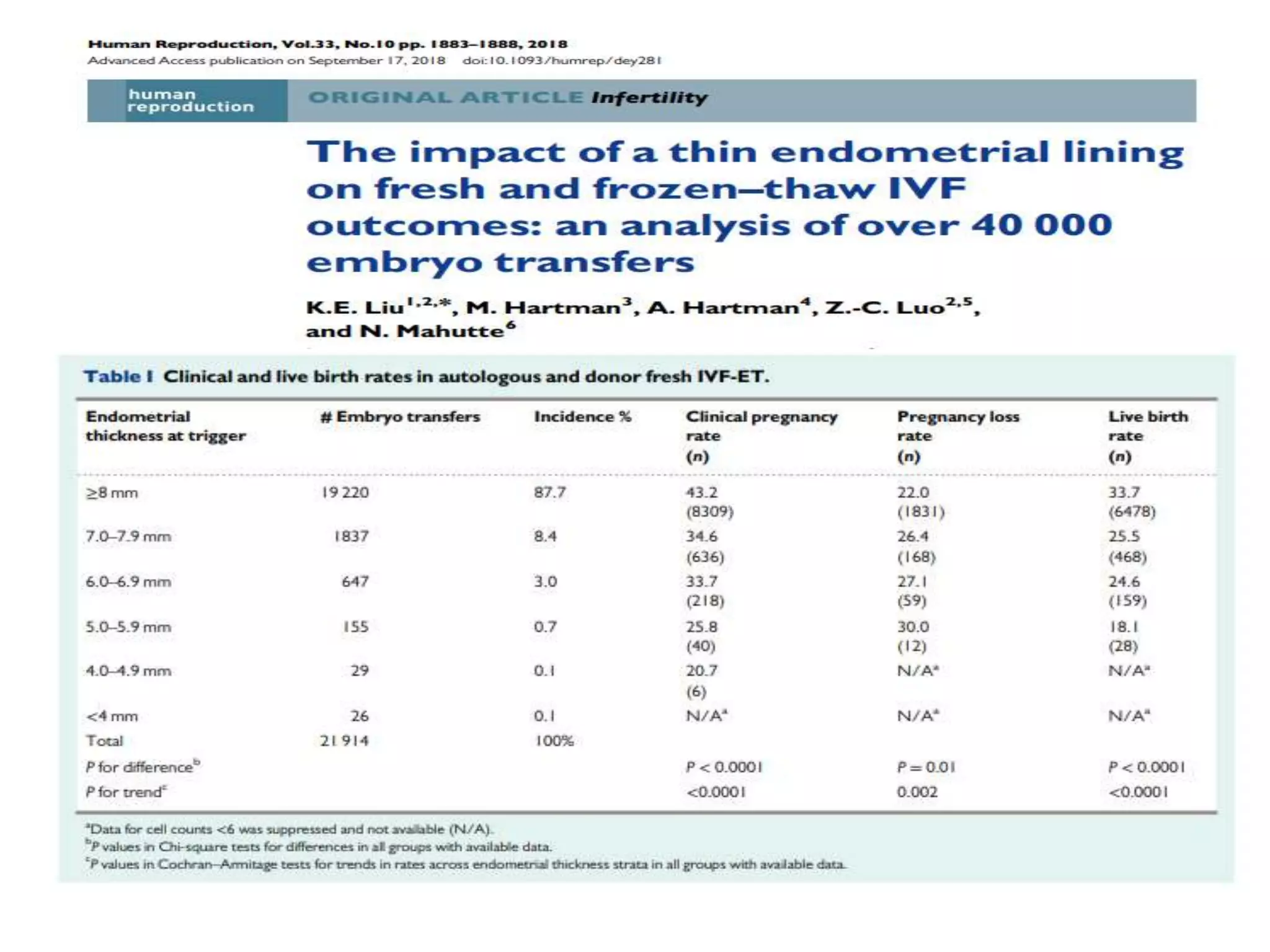
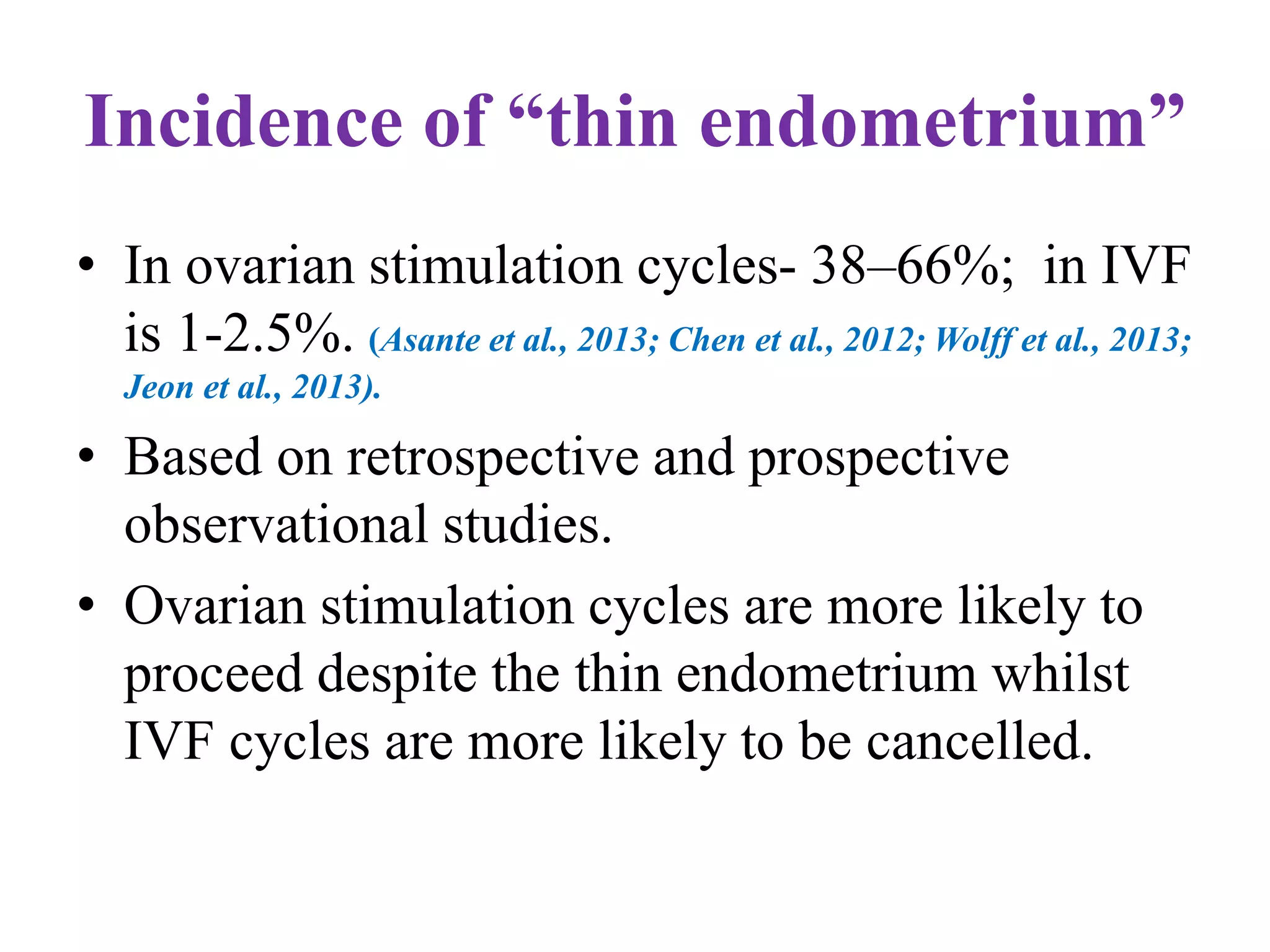
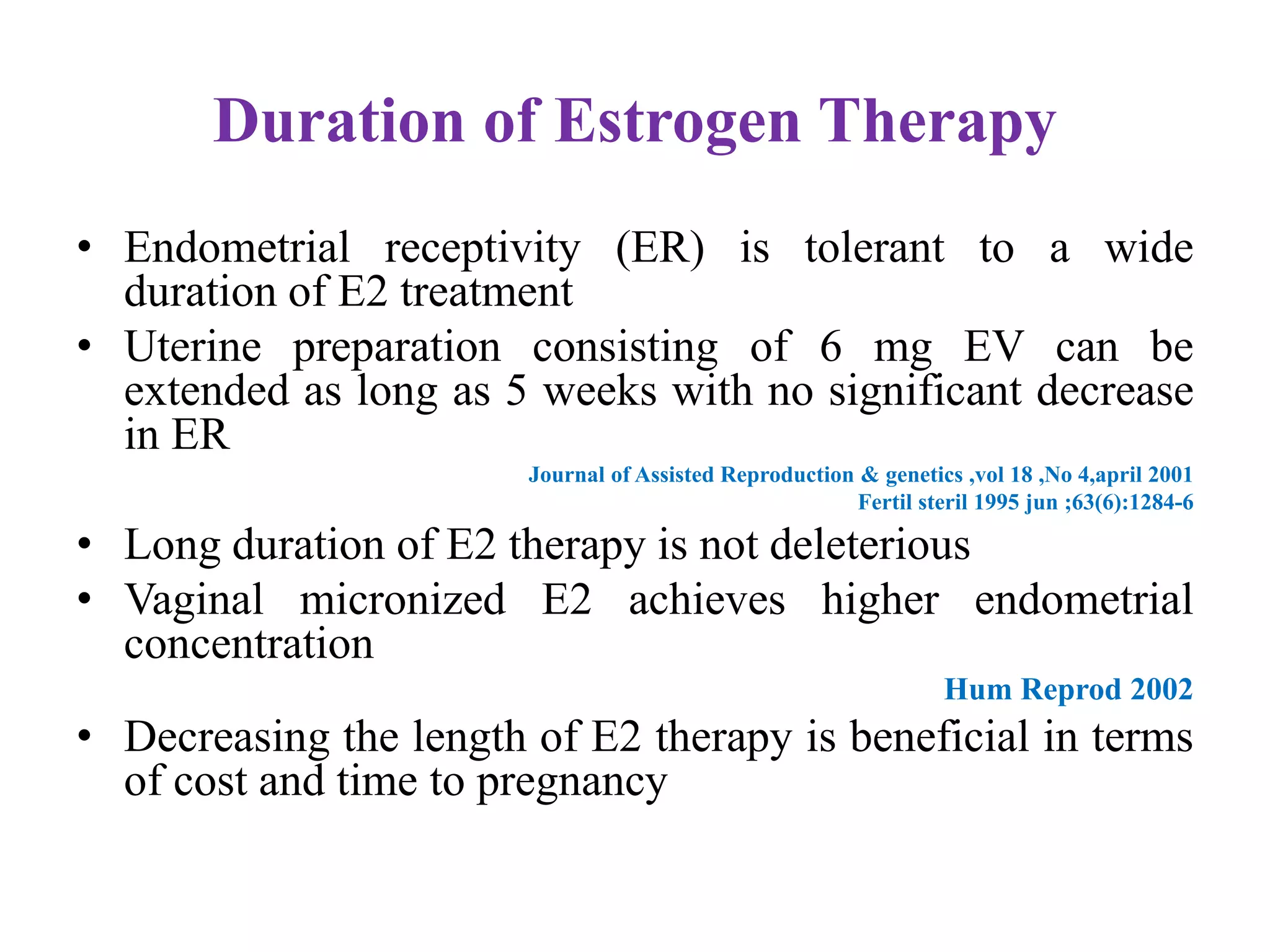
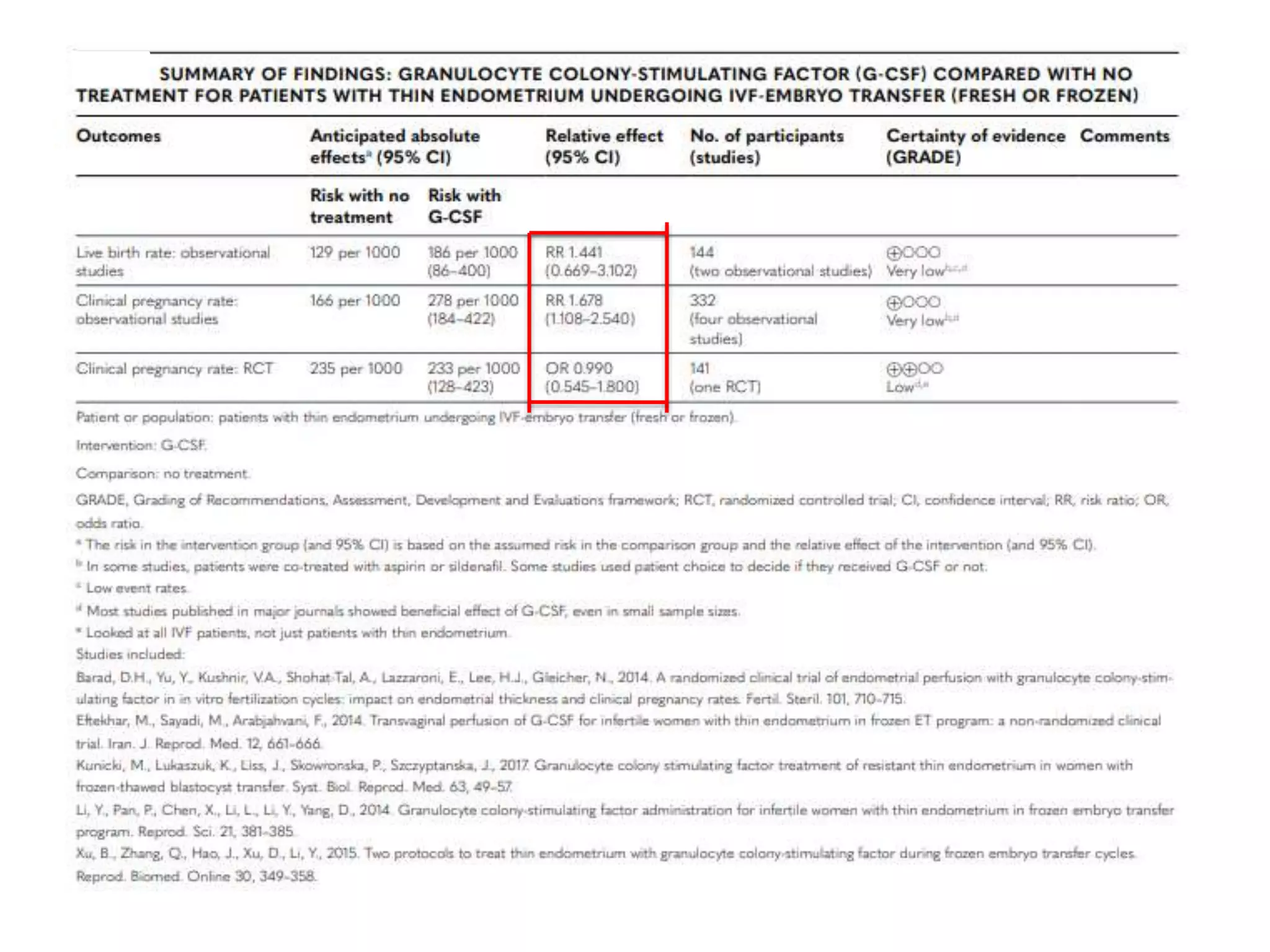
Dr. Sujoy Dasgupta, a leading expert in reproductive medicine, discusses thin endometrium and its implications for IVF success rates, noting the critical role of endometrial thickness in pregnancy outcomes. He emphasizes that clinical pregnancy and live birth rates decrease with endometrial thickness below specific thresholds and examines causes, assessment methods, and treatment options for thin endometrium. The document synthesizes findings from various studies and guidelines, outlining both the biological mechanisms involved and recommendations for clinical practice.










































![Weiss, N.S., van Vliet, M.N., Limpens, J., Hompes, P.G.A., Lambalk, C.B.,
Mochtar, M.H., van der Veen, F., Mol, B.W.J., van Wely, M. Endometrial thickness
in women undergoing IUI with ovarian stimulation. How thick is too thin? A
systematic review and meta-analysis. Hum. Reprod. 2017; 32: 1009–1018
• Meta-analysis included 1525 women in 7
studies [2 RCT and 5cohort studies]](https://image.slidesharecdn.com/thinet-200110164052/75/Thin-Endometrium-56-2048.jpg)





