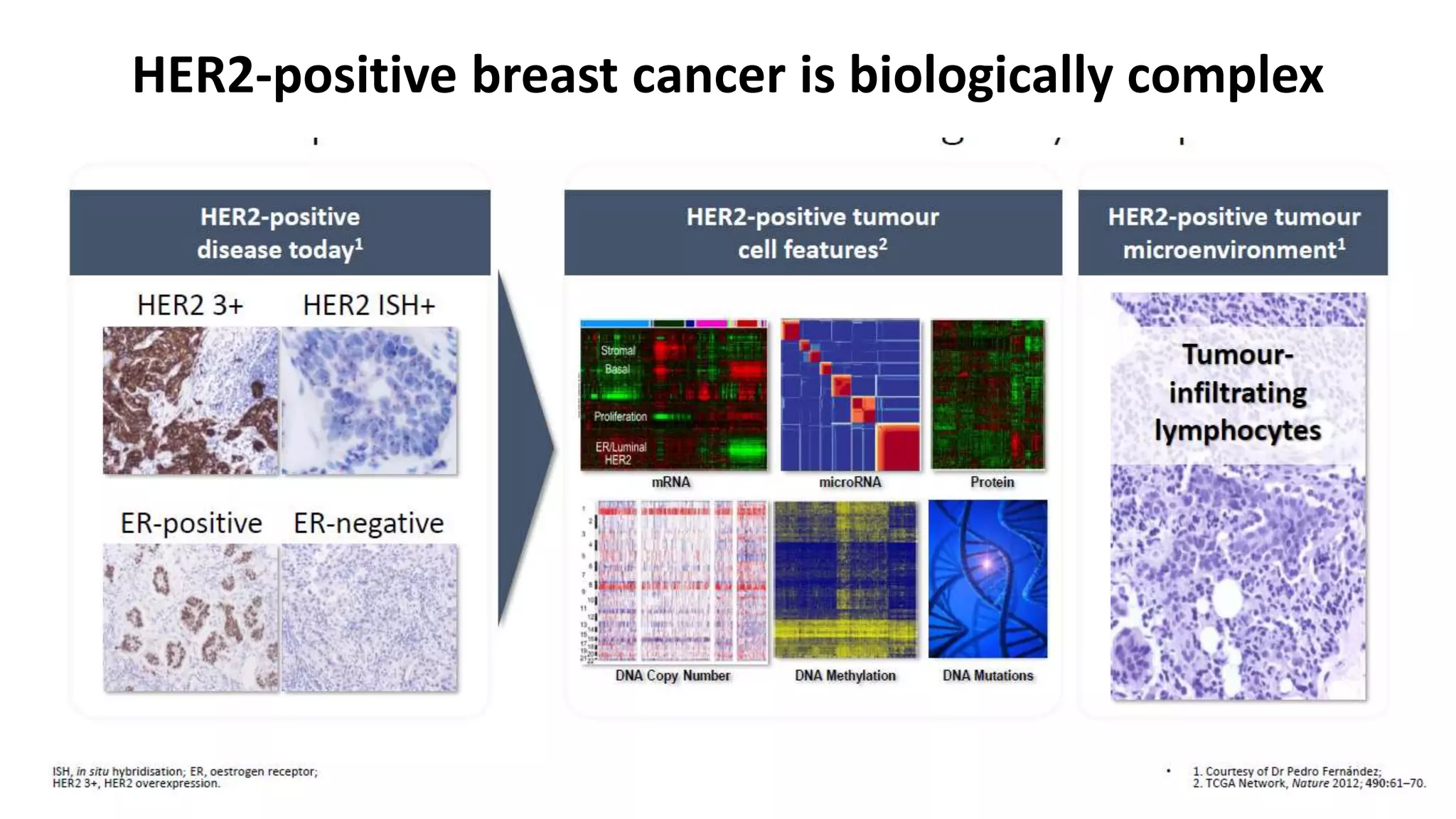
- HER2-positive early-stage breast cancer can be treated with neoadjuvant chemotherapy to shrink tumors and increase the rate of breast conservation.
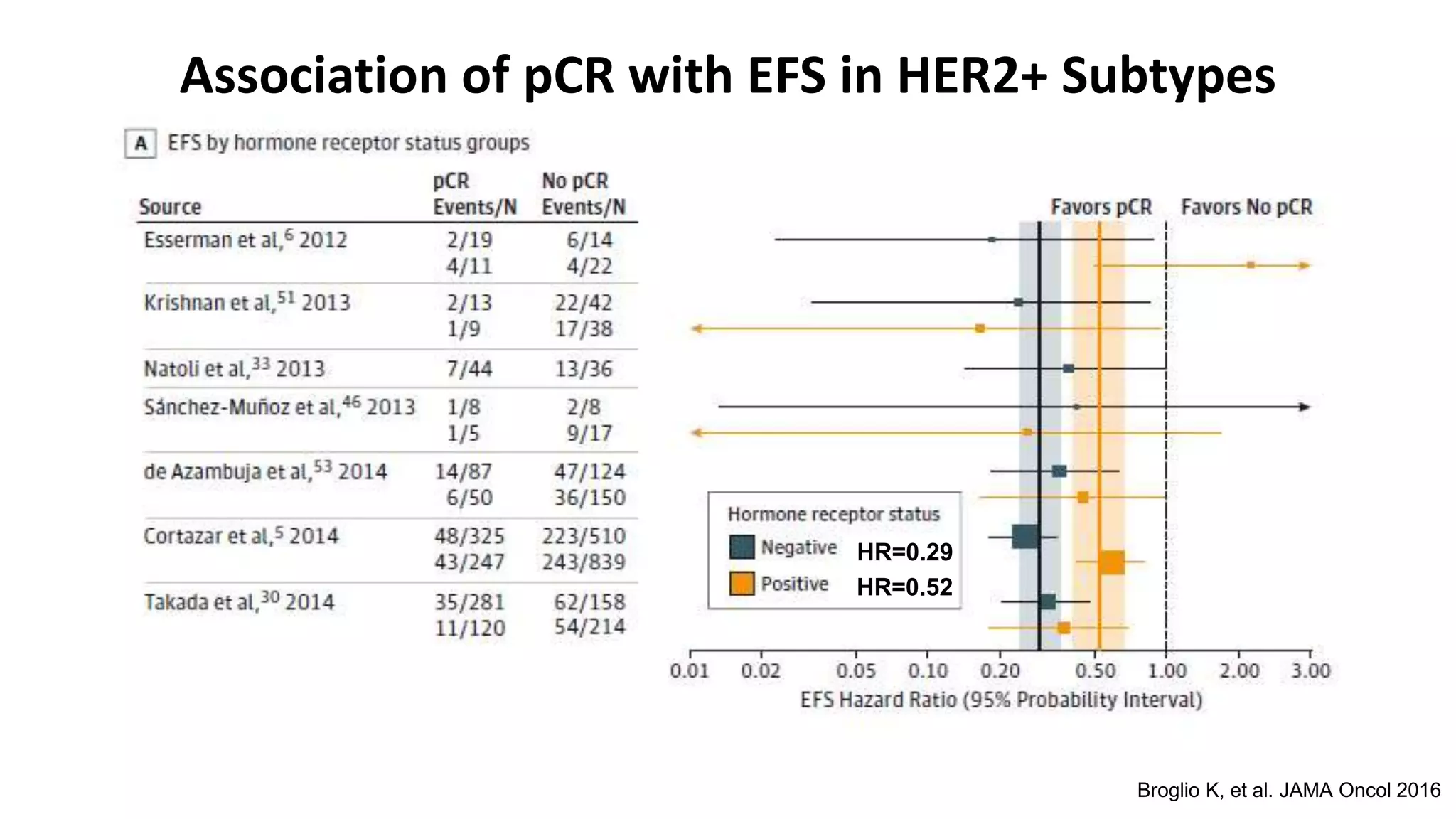
- The addition of trastuzumab to neoadjuvant chemotherapy significantly increases pathological complete response (pCR) rates. Achieving pCR correlates with improved long-term outcomes.
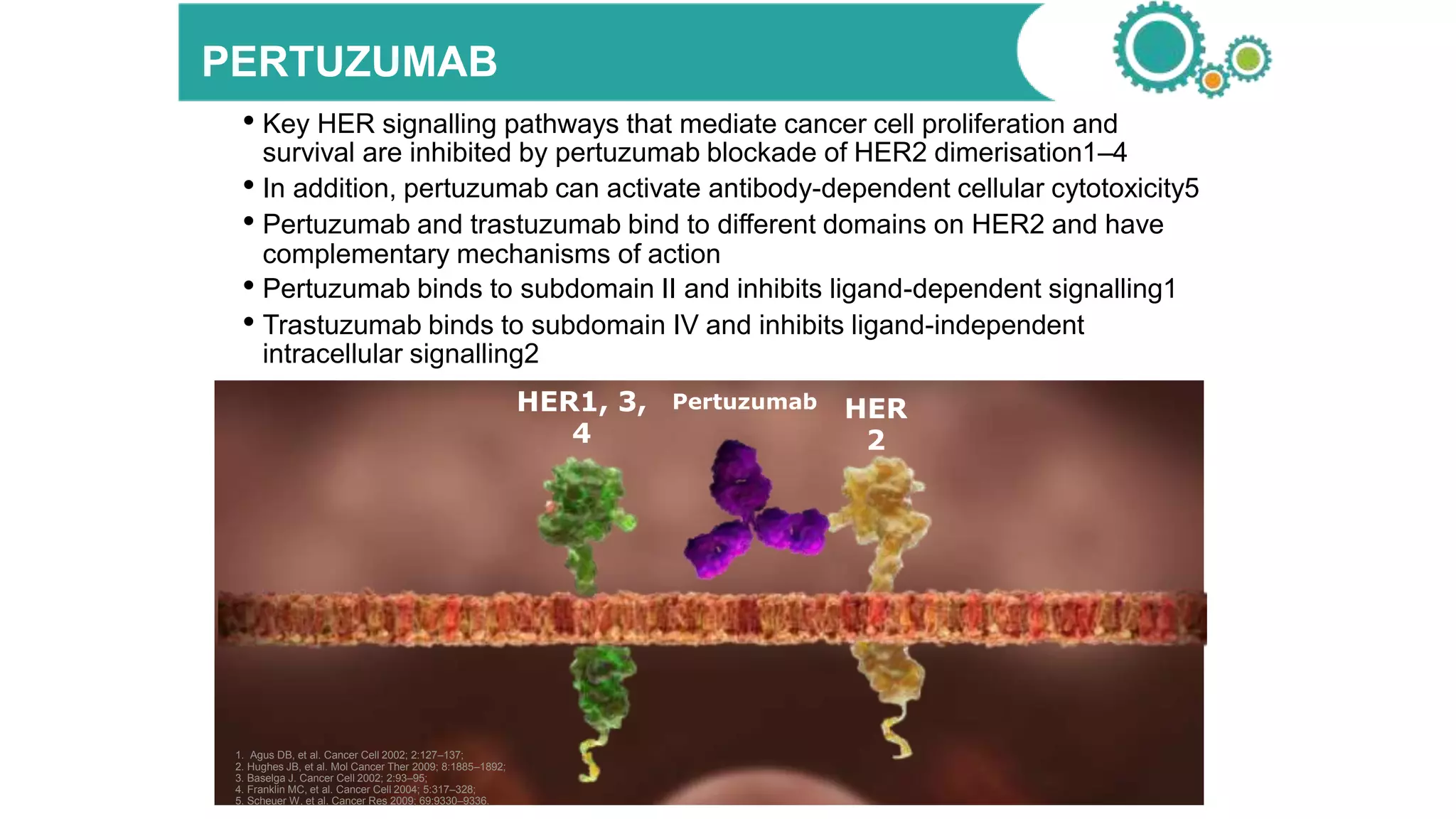
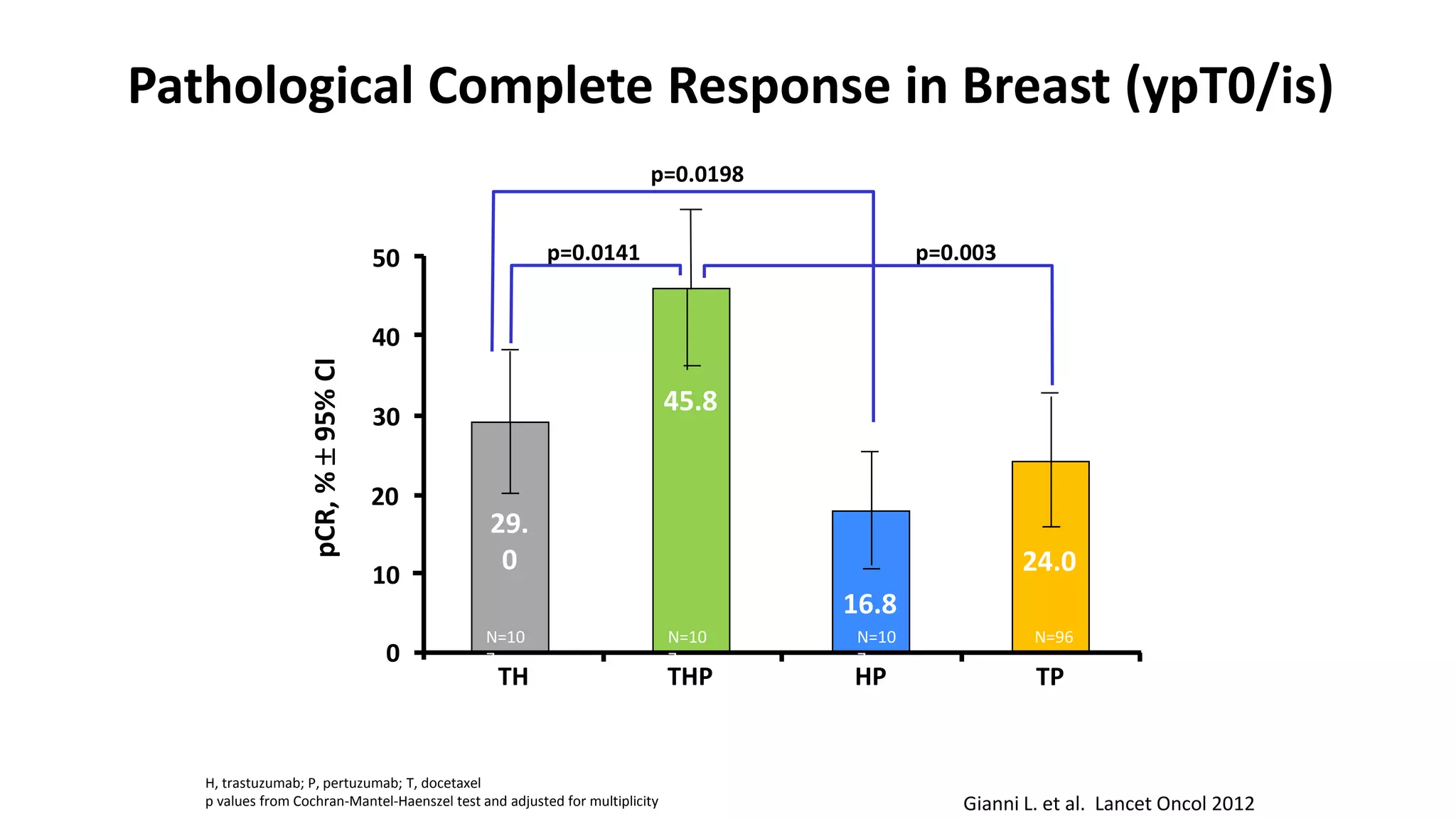
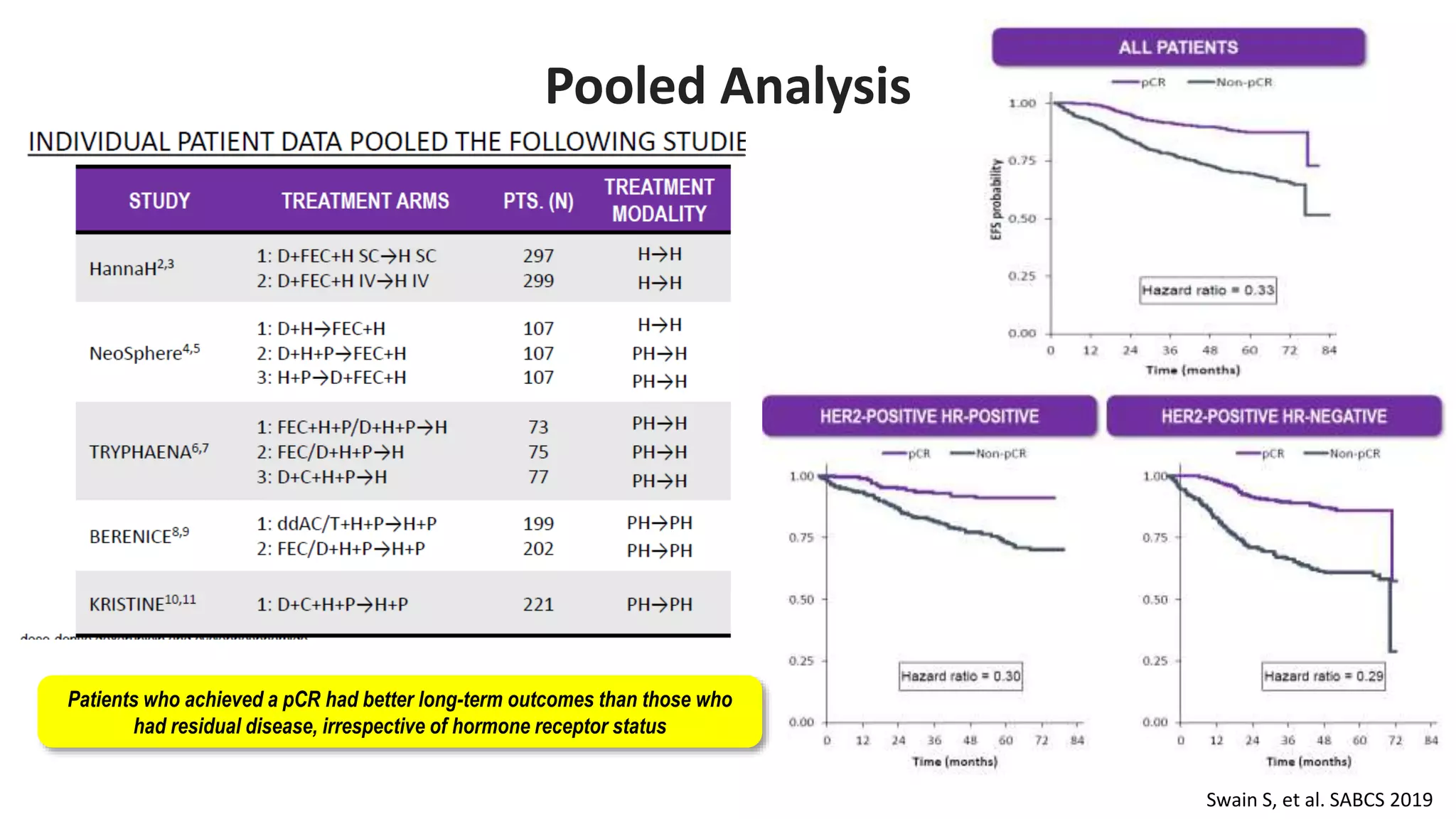
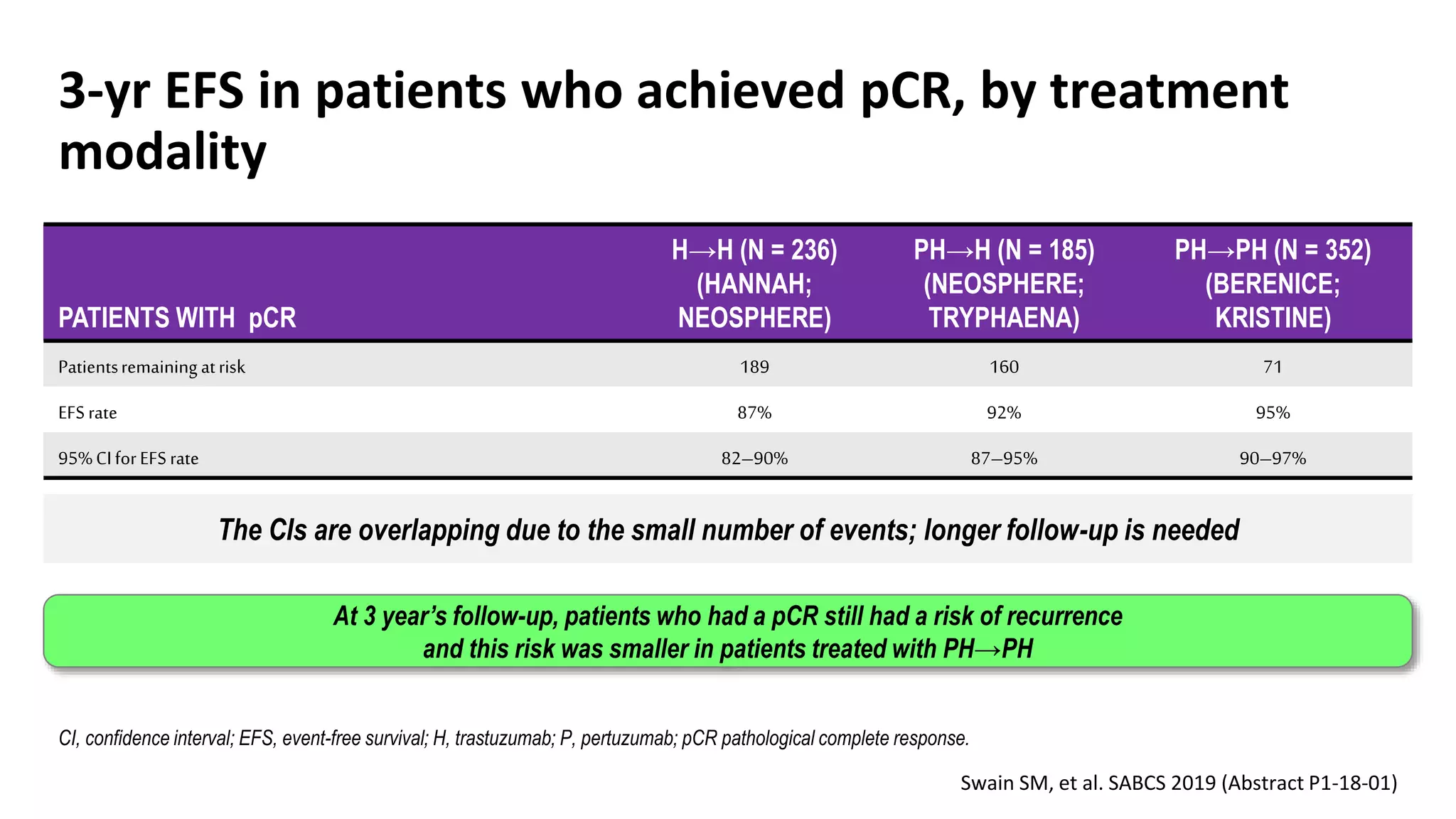
- Adding pertuzumab to trastuzumab and chemotherapy further increases pCR rates compared to chemotherapy and trastuzumab alone. Trials also suggest improved long-term outcomes with dual anti-HER2 blockade.


![Current treatment landscape for HER2 Positive breast cancer1
[1] NCCN Guidelines® for Breast Cancer Version 3.2020;
[2] https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2-positive-breast-cancer
[3] https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tucatinib-patients-her2-positive-metastatic-breast-cancer
EARLY-STAGE BREAST CANCER
Neoadjuvant Adjuvant
Chemo + Trastuzumab
+ Pertuzumab
Chemo + Trastuzumab
Chemo + Trastuzumab
+ Pertuzumab
Neratinib after adjuvant
chemo + Trastuzumab
T-DM1 for non-pCR
after neoadjuvant chemo
METASTATIC BREAST CANCER
2nd line 3rd line and beyond1st line
Taxane +
Trastuzumab
+ Pertuzumab
Capecitabine + Lapatinib/
Trastuzumab
Trastuzumab deruxtecan
(T-DXd)2
T-DM1
Lapatinib + Trastuzumab
Capecitabine + Neratinib
Tucatinib + Capecitabine +
Trastuzumab3
ESMO Guidelines
do not reflect the
latest agents in 3rd
line treatment as
they are only
approved in the
US2,3](https://image.slidesharecdn.com/her2nact-aug20-copy-200905150609/75/Her2-nact-aug-20-copy-4-2048.jpg)