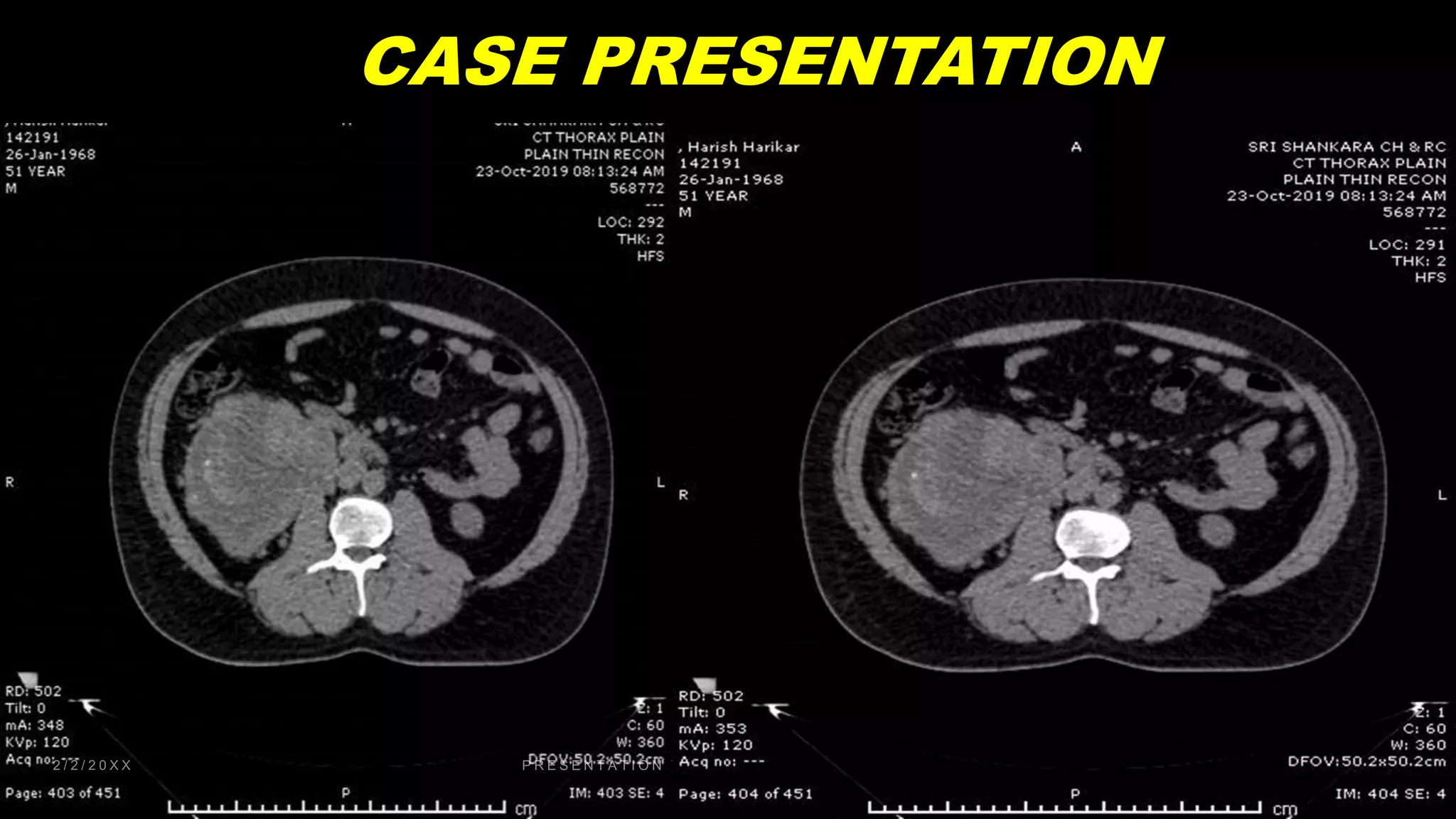
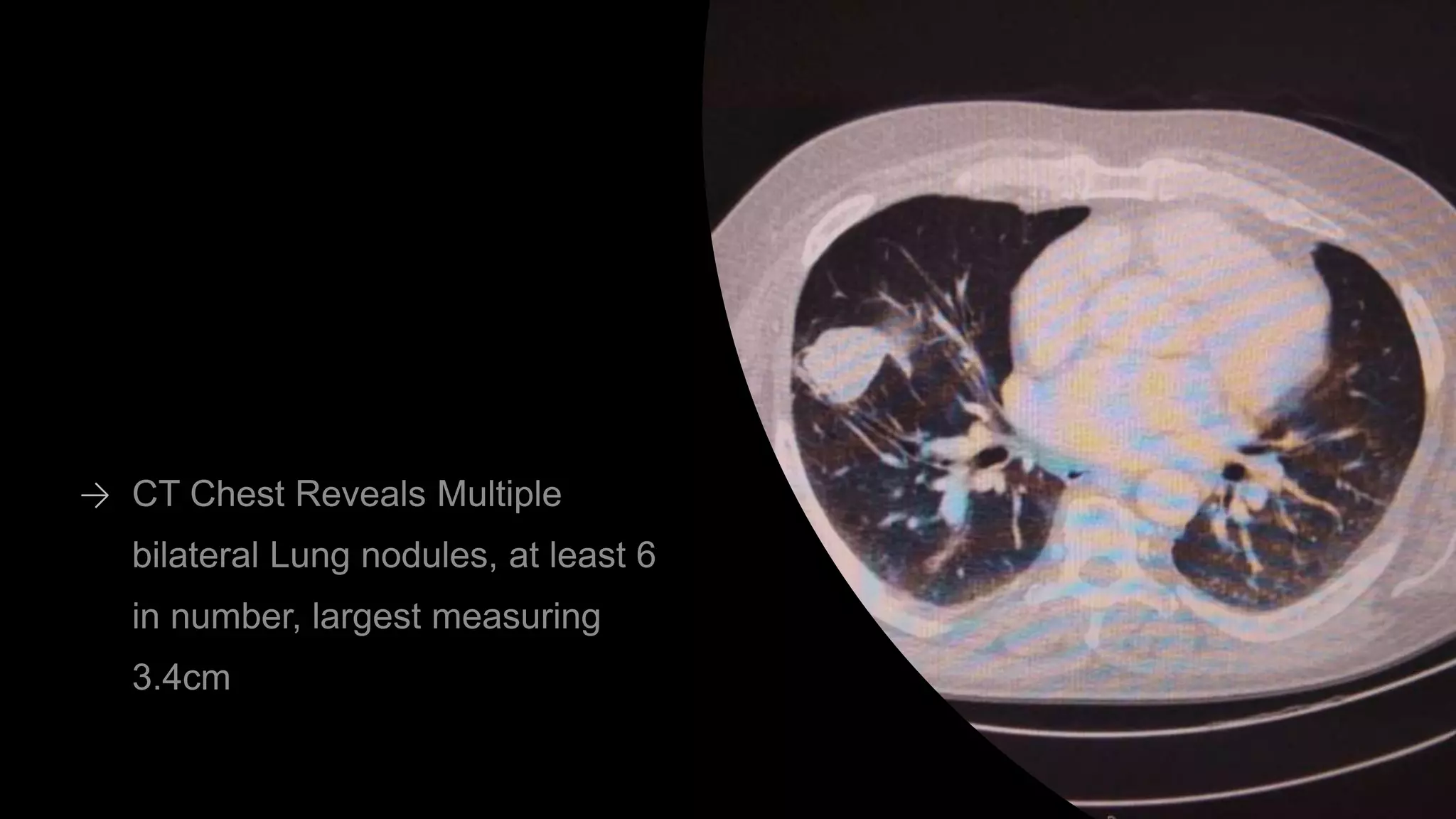
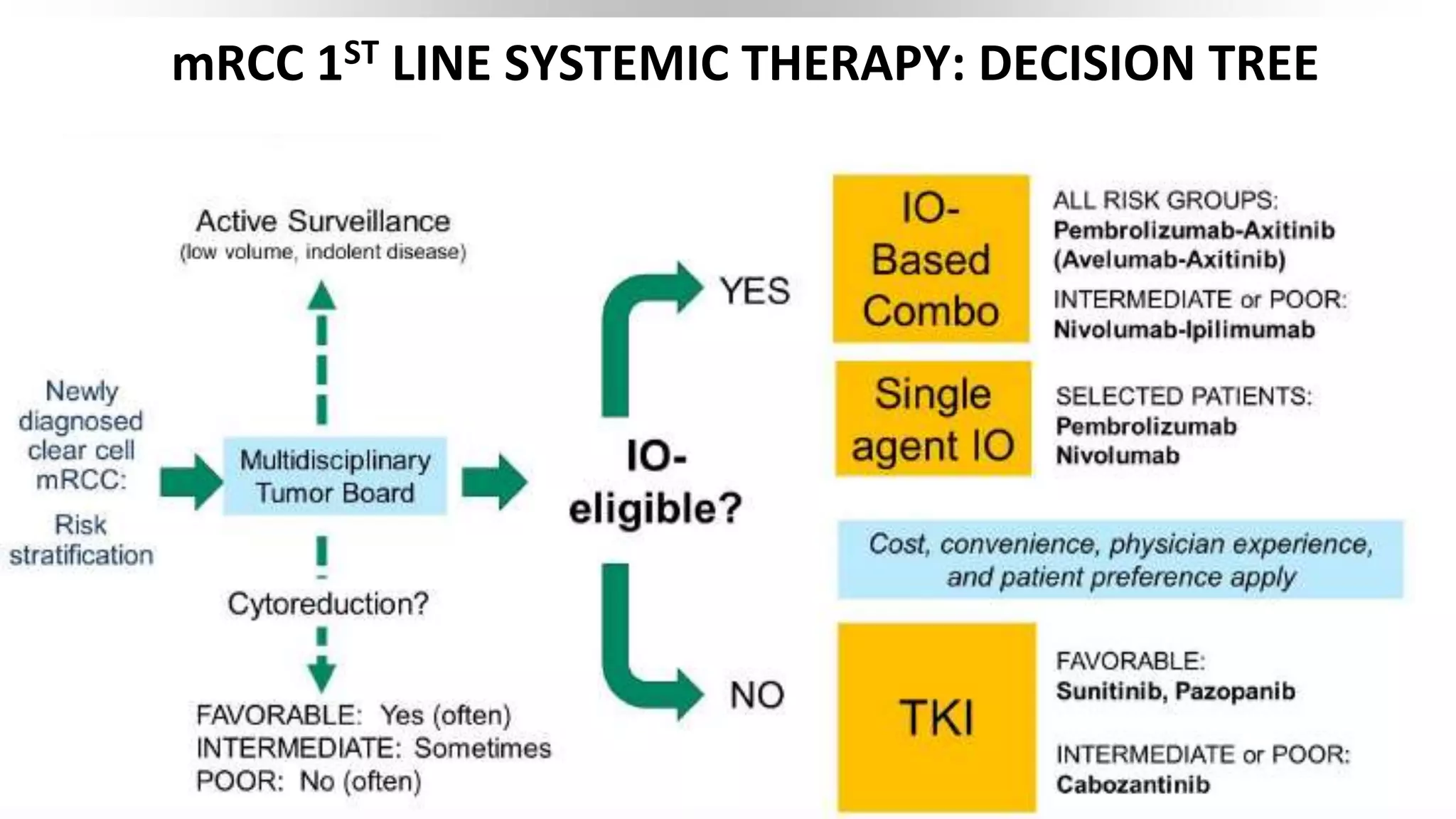
This document discusses a clinical case presentation of a patient with metastatic renal cell carcinoma (mRCC). Key details include that the patient previously underwent nephrectomy and radiation therapy and is now being discussed for systemic therapy options. The document reviews several clinical trials evaluating different combination regimens for first-line and subsequent lines of treatment in mRCC. Factors like prognostic risk categories and biomarkers are discussed for guiding treatment selection. The merits and limitations of different studies are evaluated.
























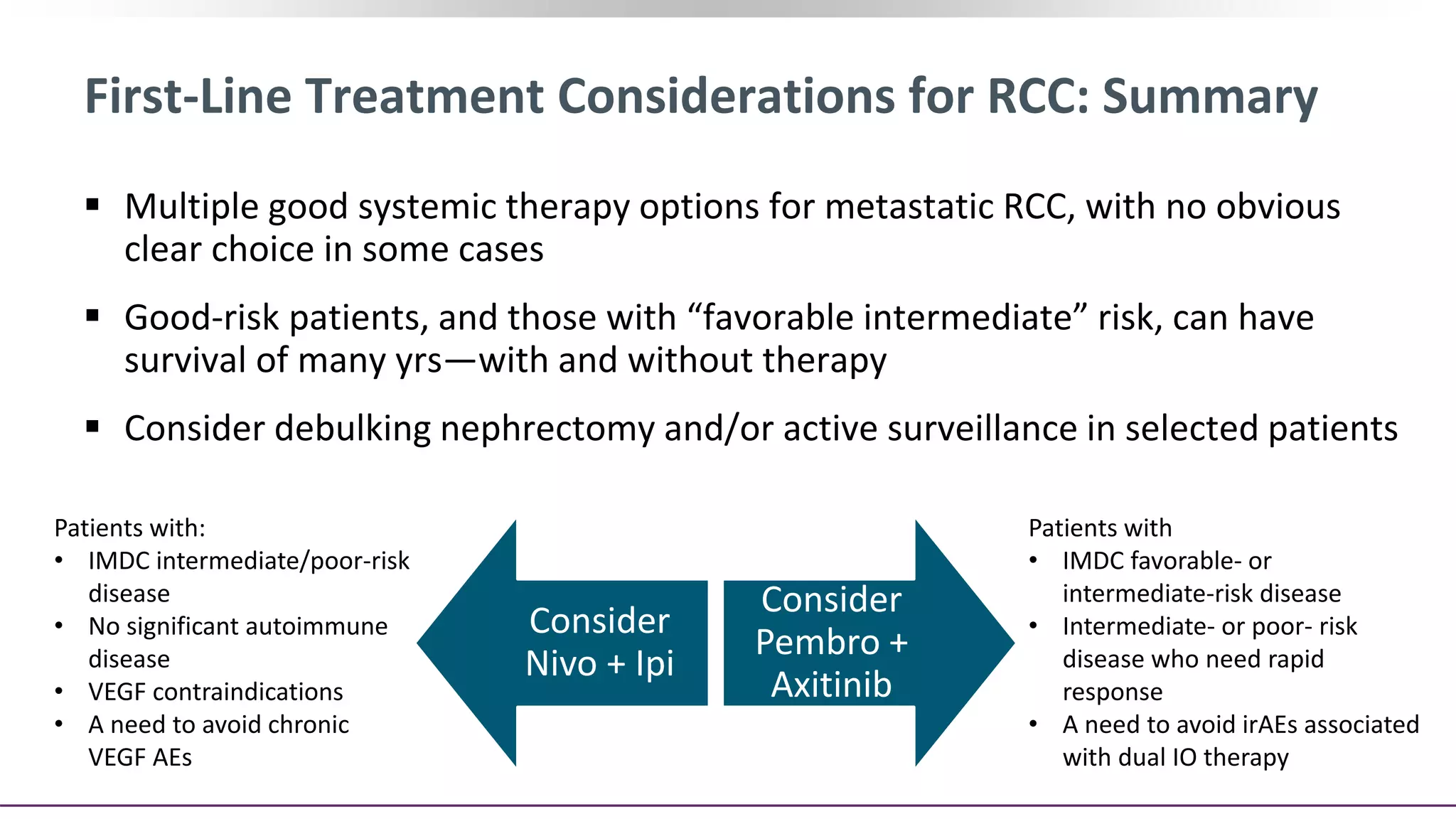







![Pivotal Randomized Trials in Clear-Cell RCC
Post TKI Therapy
Parameter
RECORD-1[1,2] AXIS[3,4] METEOR[5,6] CheckMate 025[7] LEN EVE[8]
Everolimus
vs
Placebo
Axitinib
vs
Sorafenib
Cabozantinib
vs
Everolimus
Nivolumab
vs
Everolimus
Lenvatinib + Everolimus
vs
Everolimus
Patients, n 410 723 658 821 153
MSKCC risk, %
Good 29 28 46 36 23
Intermediate 56 37 42 49 36
Poor 15 33 13 15 40
Prior TKI Anti-VEGF Sunitinib Anti-VEGF Anti-VEGF Anti-VEGF
Line of therapy 2nd or beyond 2nd 2nd or beyond 2nd or 3rd 2nd
ORR, % 2 vs 0 19 vs 9 21 vs 5 25 vs 5 43 vs 3
Median OS, mos 14.8 vs 14.0 20.1 vs 19.2 21.4 vs 17.1 25.0 vs 19.6 25.5 vs 15.4
Slide credit: clinicaloptions.com
1. Motzer. Lancet. 2008;372:449. 2. Motzer. Cancer 2010;116:4256. 3. Rini. Lancet. 2011;378:1931. 4. Motzer. Lancet Oncol. 2013;14:552.
5. Choueiri. NEJM. 2015;373:1814. 6. Motzer. Br J Cancer. 2018;118:1176. 7. Motzer. NEJM. 2015;373:1803. 8. Motzer. Lancet. 2015;16:1473.
Phase III Phase II](https://image.slidesharecdn.com/radaronrcc-211128155041/75/Radar-on-rcc-41-2048.jpg)


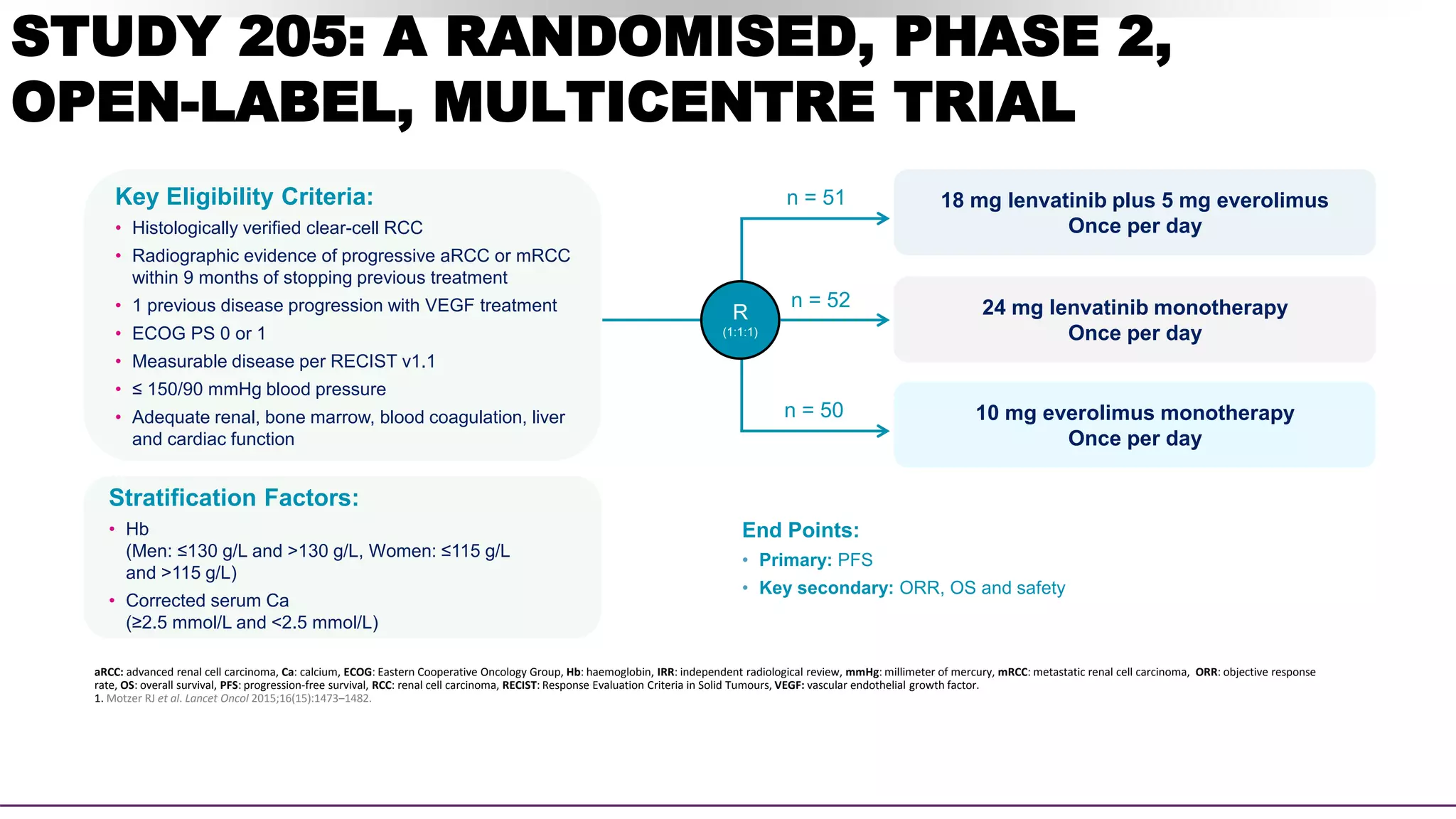

![SECONDARY ENDPOINT – ORR
Assessed by Investigator review per RECIST v1.1
CI.
Lenvatinib plus
everolimus
(n=51)
Lenvatinib
monotherapy
(n=52)
Everolimus
monotherapy
(n=50)
OBJECTIVE RESPONSE
Events 22 (43%) 14 (27%) 3 (6%)
95% CI 29–58 16–41 1–17
Best overall response
Complete
response
1 (2%) 0 0
Partial response 21 (41%) 14 (27%) 3 (6%)
Stable disease 21 (41%) 27 (52%) 31 (62%)
Progressive
disease
2 (4%) 3 (6%) 12 (24%)
Not assessed 6 (12%) 8 (15%) 4 (8%)
43% ORR
for lenvatinib plus
everolimus vs 6% with
everolimus alone
(rate ratio [RR] 7·2, 95% CI:
2·3–22·5; p<0·0001)1
Lenvatinib plus everolimus
• Duration of response was
13.0 months1
• Time to response was 1.9
months2](https://image.slidesharecdn.com/radaronrcc-211128155041/75/Radar-on-rcc-46-2048.jpg)