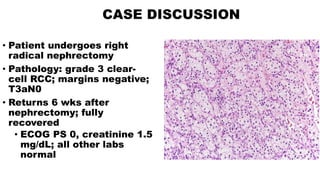
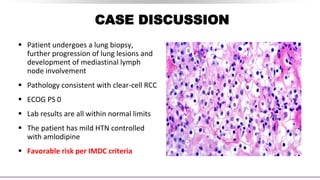
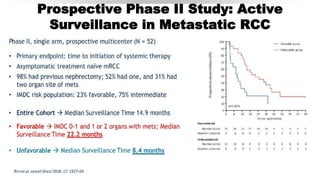
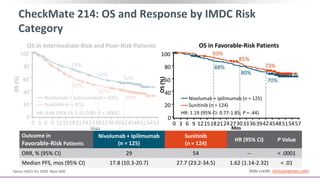
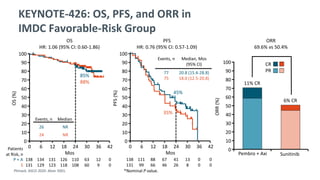
This document discusses a case of a 64-year-old man presenting with right flank pain and a history of smoking who is found to have clear-cell renal cell carcinoma (RCC). He undergoes a right radical nephrectomy and pathology confirms grade 3 clear-cell RCC without margins or lymph node involvement. Small lung nodules are detected 18 months later and biopsy confirms metastatic clear cell RCC. Systemic therapy options for the metastatic disease are discussed, including tyrosine kinase inhibitors, immunotherapy, and their combinations. Ongoing trials of immunotherapy in the adjuvant and metastatic settings are also summarized. Risk stratification models and their impact on treatment selection are reviewed.





![PUBLISHED TYROSINE KINASE INHIBITOR
ADJUVANT TRIALS
Trial Therapy N Histology Stage
Starting
Dose
Minimum
Dose
DFS OS
ASSURE[1] Sunitinib
Sorafenib
Placebo
1943 79% ccRCC
> pT1b, G3-4,
or N+
50 or 37.5
mg (Su)/
400 mg
(So)
25 mg
(Su)/40 mg
(So)
No No
S-TRAC[2] Sunitinib
Placebo
615 ccRCC > pT3b or N+ 50 mg 37.5 mg Yes No
PROTECT[3] Pazopanib
Placebo
1538
ccRCC or
mostly
ccRCC
pT2 (G3-4), ≥
pT3, or N+
600 mg 400 mg No No](https://image.slidesharecdn.com/paneldiscussiononarcc-210321143518/85/Panel-discussion-on-a-rcc-9-320.jpg)
![ONGOING PHASE III ADJUVANT TRIALS:
IMMUNOTHERAPY VS PLACEBO
Parameter
IMmotion010[1]
(NCT03024996)
PROSPER[2] (NCT03055013)
KEYNOTE-564[3]
(NCT03142334)
CheckMate 914[4]
(NCT03138512)
Drug Atezolizumab Nivolumab Pembrolizumab Nivolumab + ipilimumab
Histology
Clear-cell ± sarcomatoid
histology
RCC of any histology
Clear-cell ± sarcomatoid
features
Clear-cell ± sarcomatoid
features
Dose duration 1 yr
2 doses prior to surgery and
adjuvant nivolumab for 9
mos
1 yr 6 mos
Risk
classification
T2 grade 4, T3a grade 3/4,
T3b/c any grade, T4 any grade,
or TxN+ any grade
Clinical stage ≥ T2 or
any N+
pT2, grade 4; pT3/4, any
grade; N+ M0; M1 NED
pT2aN0, grade 3-4; pT2b-T4;
N+
Primary
endpoint
DFS RFS at 5 yrs DFS DFS
BICR Yes Yes Yes Yes
Status Active, recruiting Active, recruiting Active, recruiting Active, recruiting
1.](https://image.slidesharecdn.com/paneldiscussiononarcc-210321143518/85/Panel-discussion-on-a-rcc-10-320.jpg)


















![Pivotal Randomized Trials in Clear-Cell RCC
Post TKI Therapy
Parameter
RECORD-1[1,2] AXIS[3,4] METEOR[5,6] CheckMate 025[7] LEN EVE[8]
Everolimus
vs
Placebo
Axitinib
vs
Sorafenib
Cabozantinib
vs
Everolimus
Nivolumab
vs
Everolimus
Lenvatinib + Everolimus
vs
Everolimus
Patients, n 410 723 658 821 153
MSKCC risk, %
Good 29 28 46 36 23
Intermediate 56 37 42 49 36
Poor 15 33 13 15 40
Prior TKI Anti-VEGF Sunitinib Anti-VEGF Anti-VEGF Anti-VEGF
Line of therapy 2nd or beyond 2nd 2nd or beyond 2nd or 3rd 2nd
ORR, % 2 vs 0 19 vs 9 21 vs 5 25 vs 5 43 vs 3
Median OS, mos 14.8 vs 14.0 20.1 vs 19.2 21.4 vs 17.1 25.0 vs 19.6 25.5 vs 15.4
Slide credit: clinicaloptions.com
1. Motzer. Lancet. 2008;372:449. 2. Motzer. Cancer 2010;116:4256. 3. Rini. Lancet. 2011;378:1931. 4. Motzer. Lancet Oncol. 2013;14:552.
5. Choueiri. NEJM. 2015;373:1814. 6. Motzer. Br J Cancer. 2018;118:1176. 7. Motzer. NEJM. 2015;373:1803. 8. Motzer. Lancet. 2015;16:1473.
Phase III Phase II](https://image.slidesharecdn.com/paneldiscussiononarcc-210321143518/85/Panel-discussion-on-a-rcc-39-320.jpg)





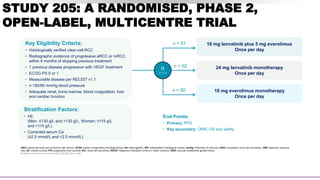
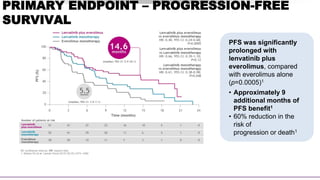
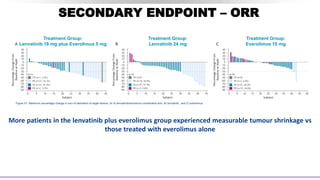
![SECONDARY ENDPOINT – ORR
Assessed by Investigator review per RECIST v1.1
CI.
Lenvatinib plus
everolimus
(n=51)
Lenvatinib
monotherapy
(n=52)
Everolimus
monotherapy
(n=50)
OBJECTIVE RESPONSE
Events 22 (43%) 14 (27%) 3 (6%)
95% CI 29–58 16–41 1–17
Best overall response
Complete
response
1 (2%) 0 0
Partial response 21 (41%) 14 (27%) 3 (6%)
Stable disease 21 (41%) 27 (52%) 31 (62%)
Progressive
disease
2 (4%) 3 (6%) 12 (24%)
Not assessed 6 (12%) 8 (15%) 4 (8%)
43% ORR
for lenvatinib plus
everolimus vs 6% with
everolimus alone
(rate ratio [RR] 7·2, 95% CI:
2·3–22·5; p<0·0001)1
Lenvatinib plus everolimus
• Duration of response was
13.0 months1
• Time to response was 1.9
months2](https://image.slidesharecdn.com/paneldiscussiononarcc-210321143518/85/Panel-discussion-on-a-rcc-49-320.jpg)