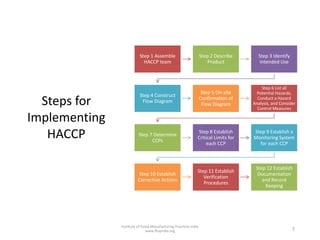
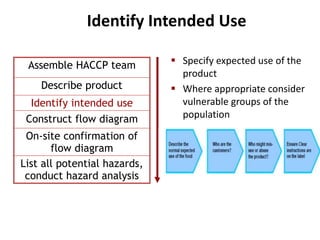
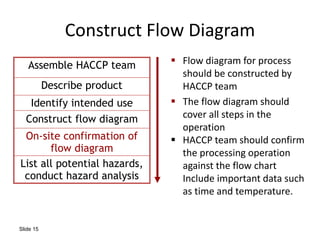
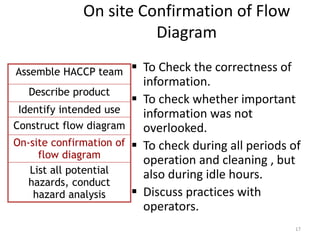
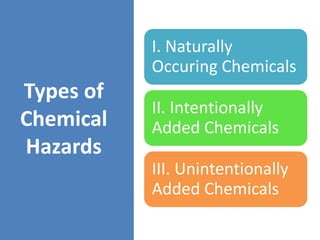
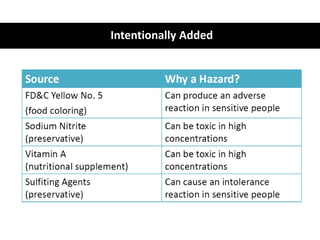
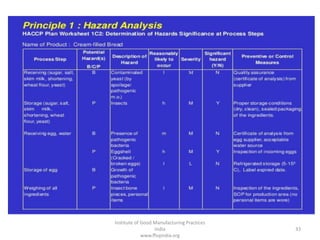
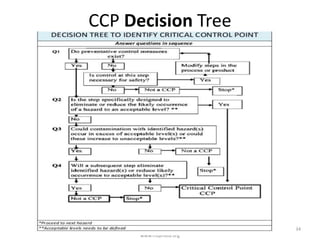
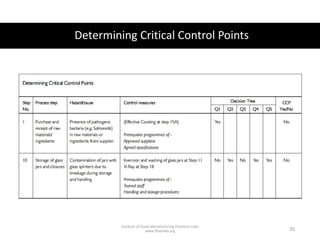
HACCP is a risk-based food safety system used by the food industry to prevent foodborne hazards. It involves identifying potential hazards at specific points in the food production process and establishing controls to prevent, eliminate, or reduce hazards to safe levels. The key principles of HACCP include conducting a hazard analysis, determining critical control points, establishing critical limits, monitoring procedures, corrective actions, verification, and documentation. The document provides details on each step of implementing a HACCP system from assembling a HACCP team to establishing record keeping procedures.