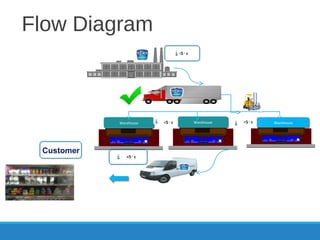
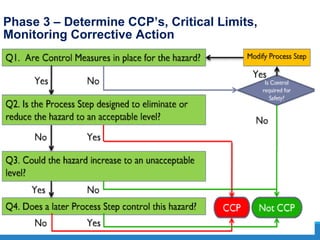
The document provides an introduction and overview of Hazard Analysis Critical Control Points (HACCP). It discusses what HACCP is, food poisoning symptoms and causes, and types of food hazards including chemical, physical, and microbiological. It also outlines key HACCP principles like conducting a hazard analysis, determining critical control points, establishing critical limits and monitoring procedures, and documenting the HACCP system. Pre-requisite programs are identified that must be in place to support an effective HACCP plan.