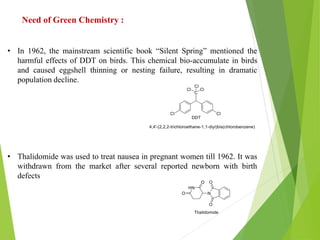
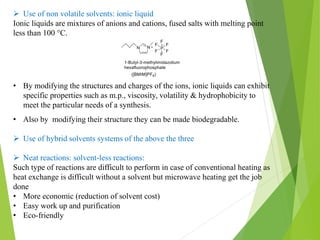
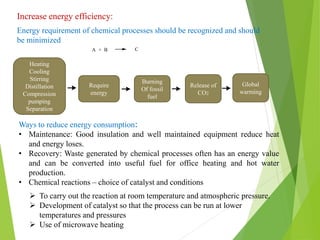
Green chemistry aims to reduce or eliminate the use of hazardous substances in chemical products and processes. It seeks to design chemical syntheses and products that are less polluting and more environmentally friendly. The principles of green chemistry encourage using safer solvents and auxiliaries like water, switching to renewable feedstocks when possible, improving energy efficiency in chemical processes, and avoiding unnecessary derivatization steps. Adopting these principles can help create chemical products and processes that are less wasteful and reduce pollution at its source.