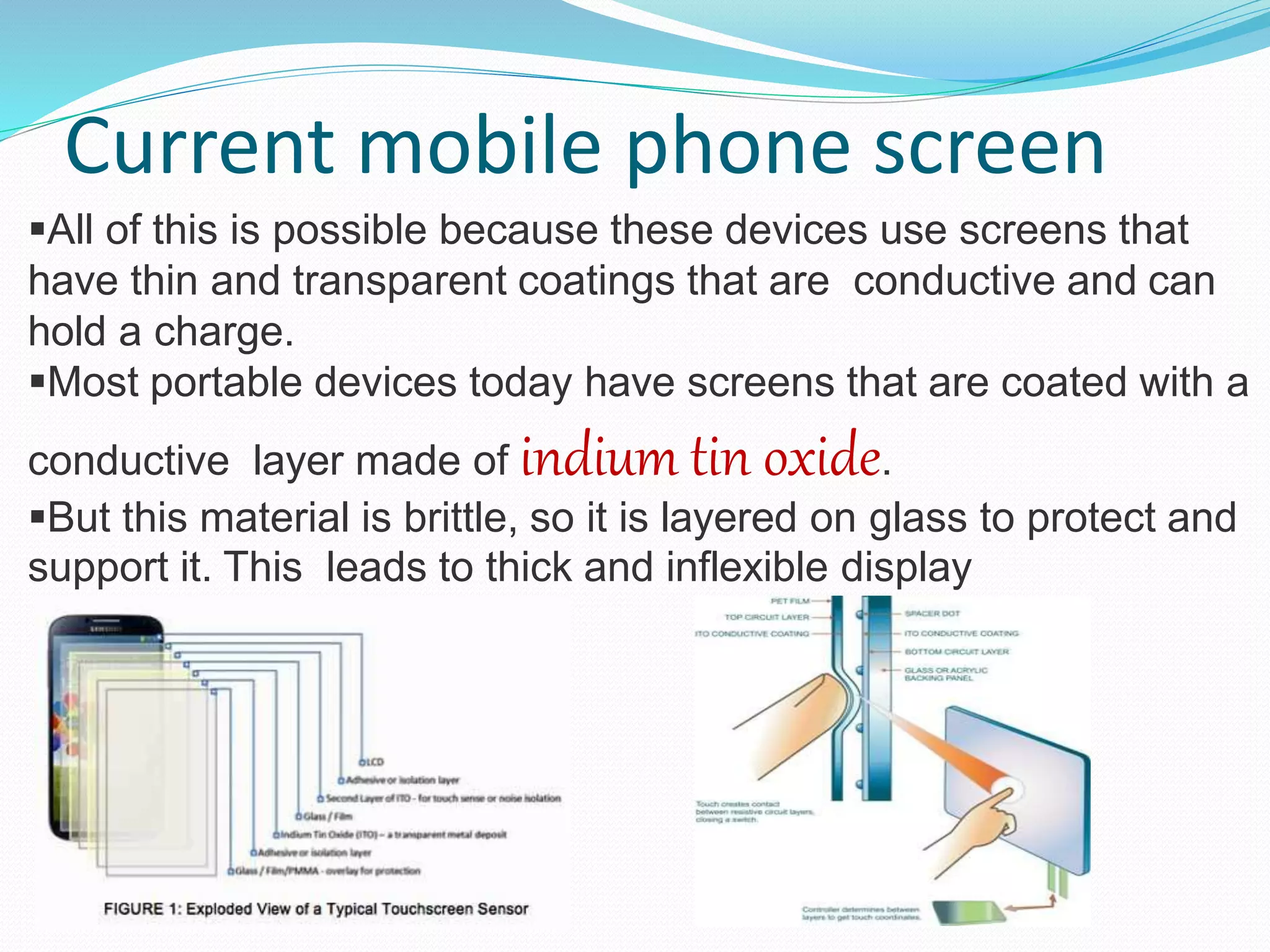
Graphene is a new wonder material that could enable many applications. It is a single layer of carbon atoms arranged in a hexagonal structure. In 2004, scientists discovered graphene's remarkable properties - it is nearly transparent, highly conductive, stronger than steel yet very light. Graphene could enable flexible touch screens, solar panels, and bionic implants. It has the potential to revolutionize many technologies and improve lives.