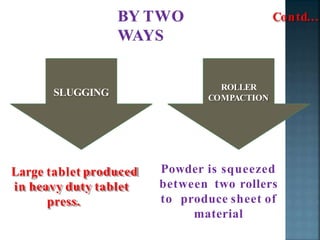
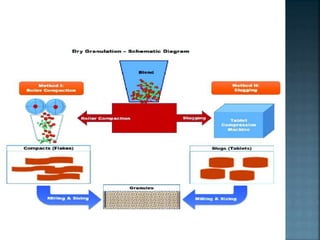
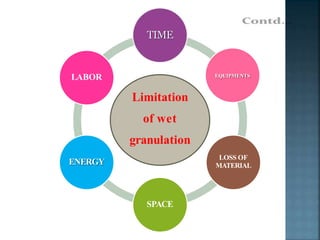
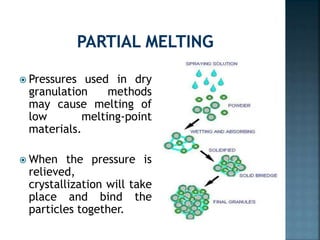
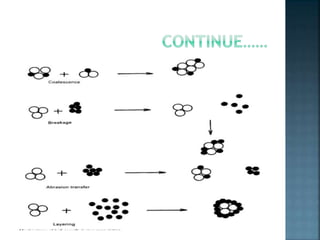
Granulation is a process that binds powder particles together into larger, multiparticle granules. It is used in pharmaceutical manufacturing to produce tablet and capsule fillers ranging from 0.2 to 4.0 mm in size. Granulation improves powder flow and uniformity, reduces dust, and enhances compaction. There are two primary types of granulation: dry granulation, which uses no liquid, and wet granulation, which uses a liquid to form granules.