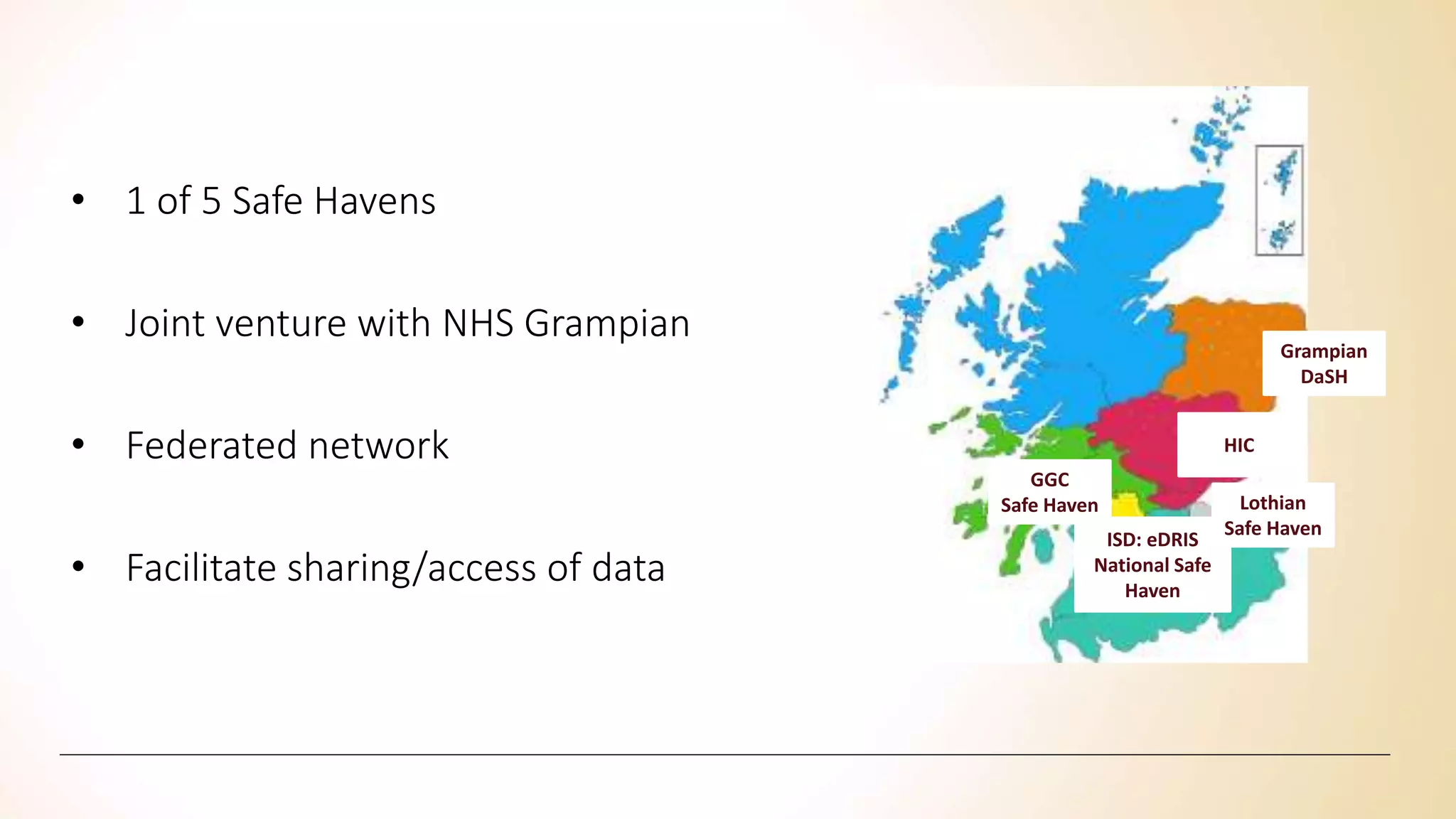
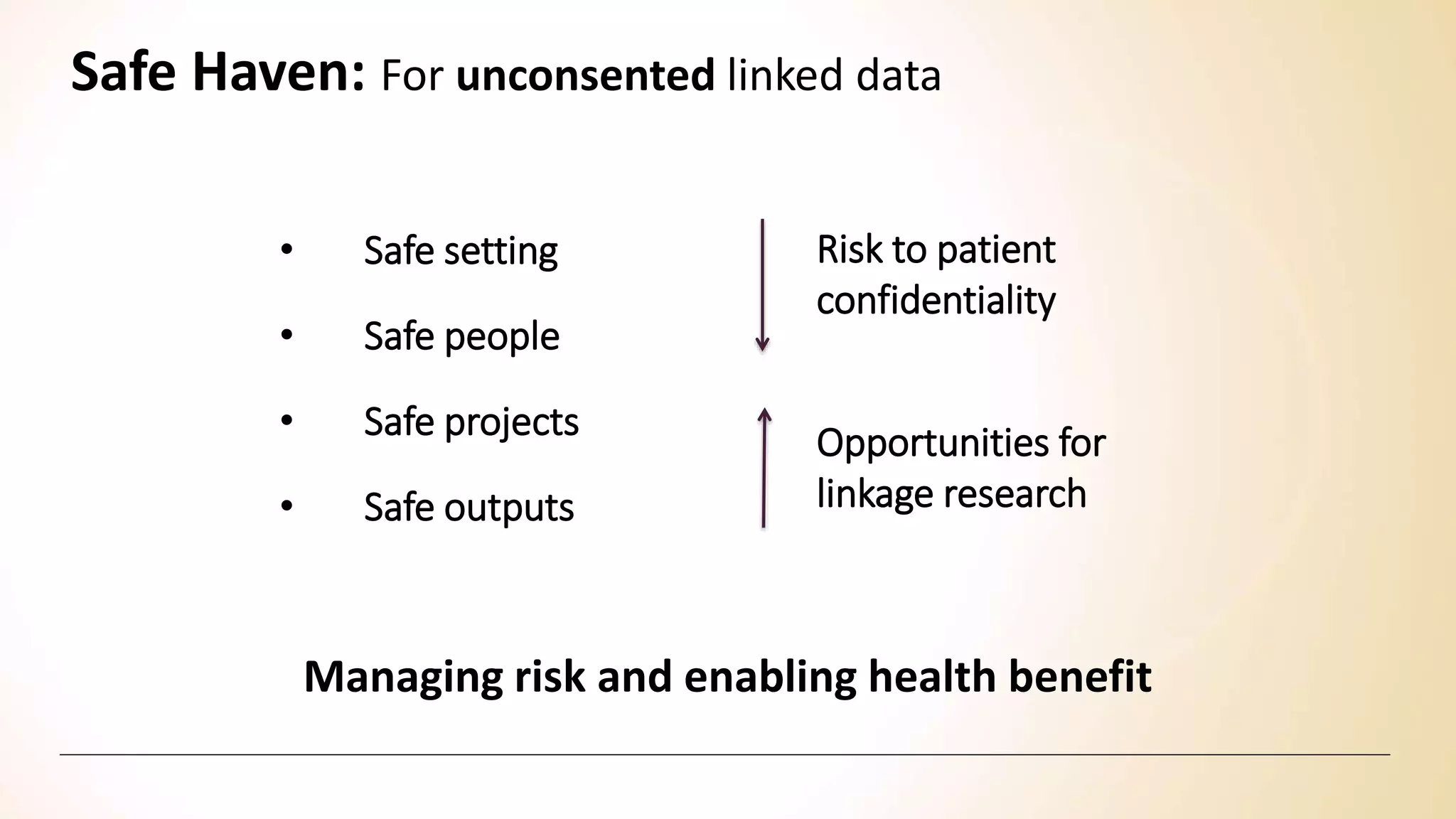
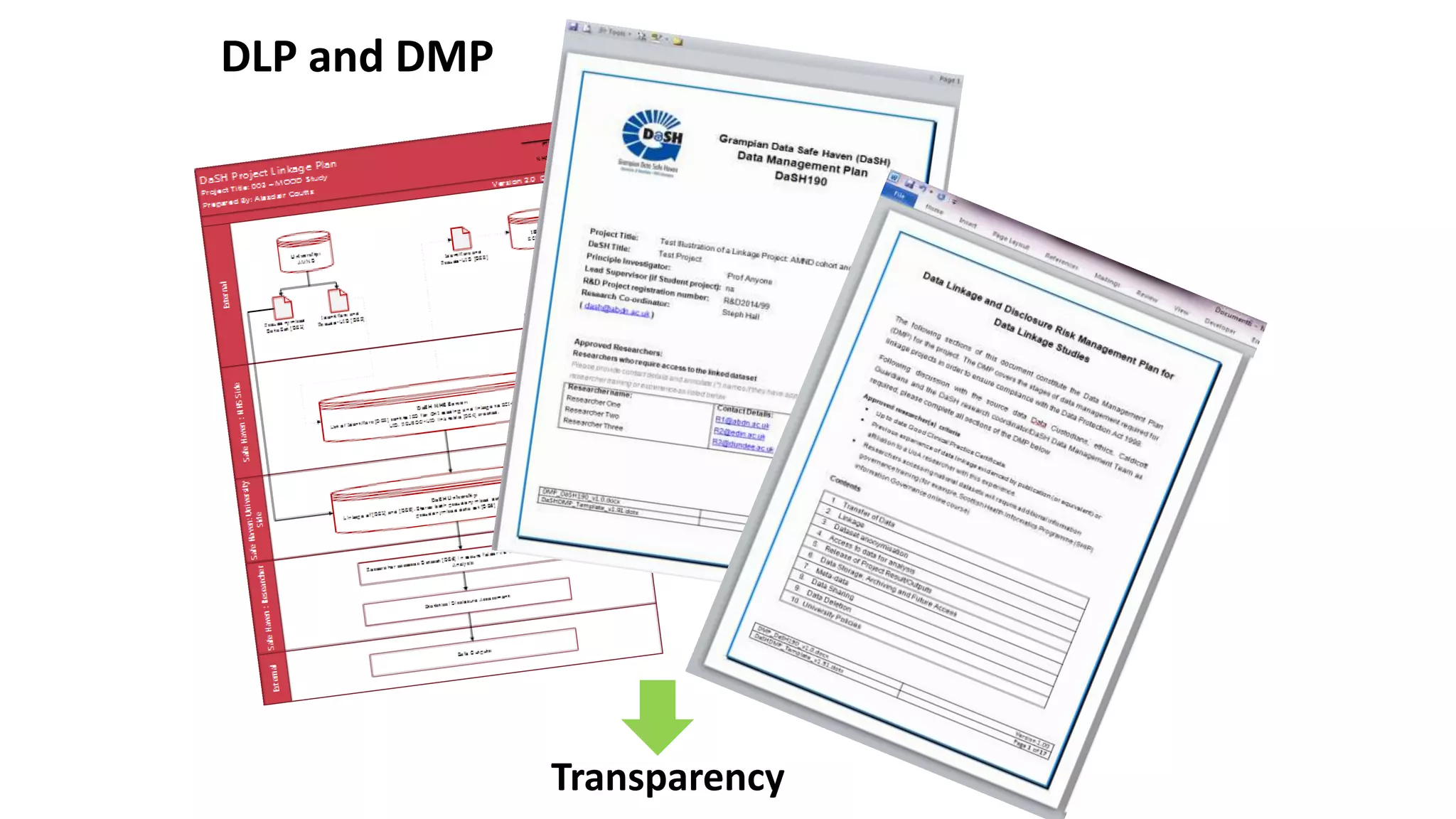
Safe havens" should be developed as an environment for population-based research where the risk of identifying individuals is minimized. Researchers in safe havens are bound by strict confidentiality codes preventing disclosure of personally identifying information and providing sanctions for breaches of confidentiality.