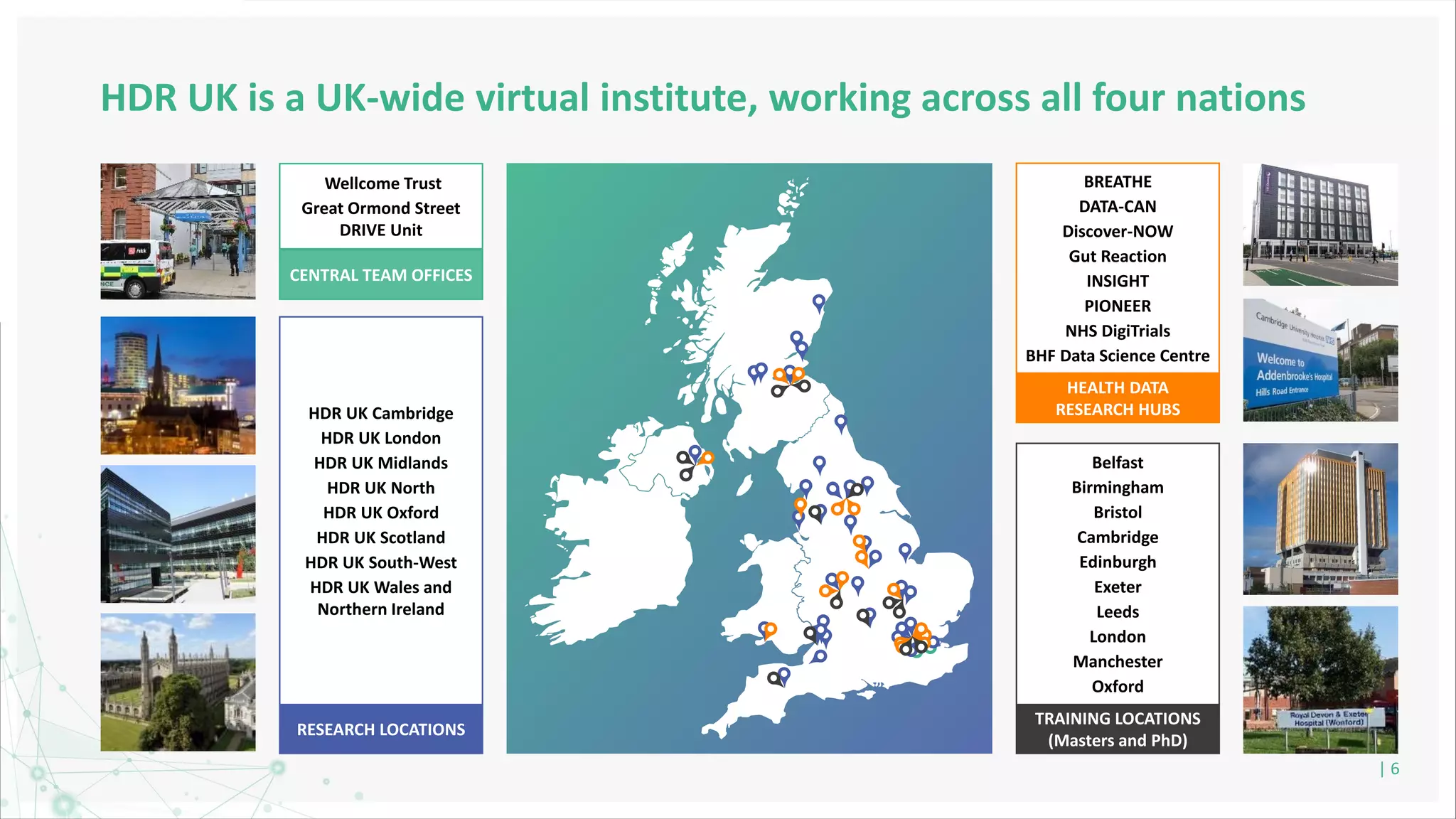
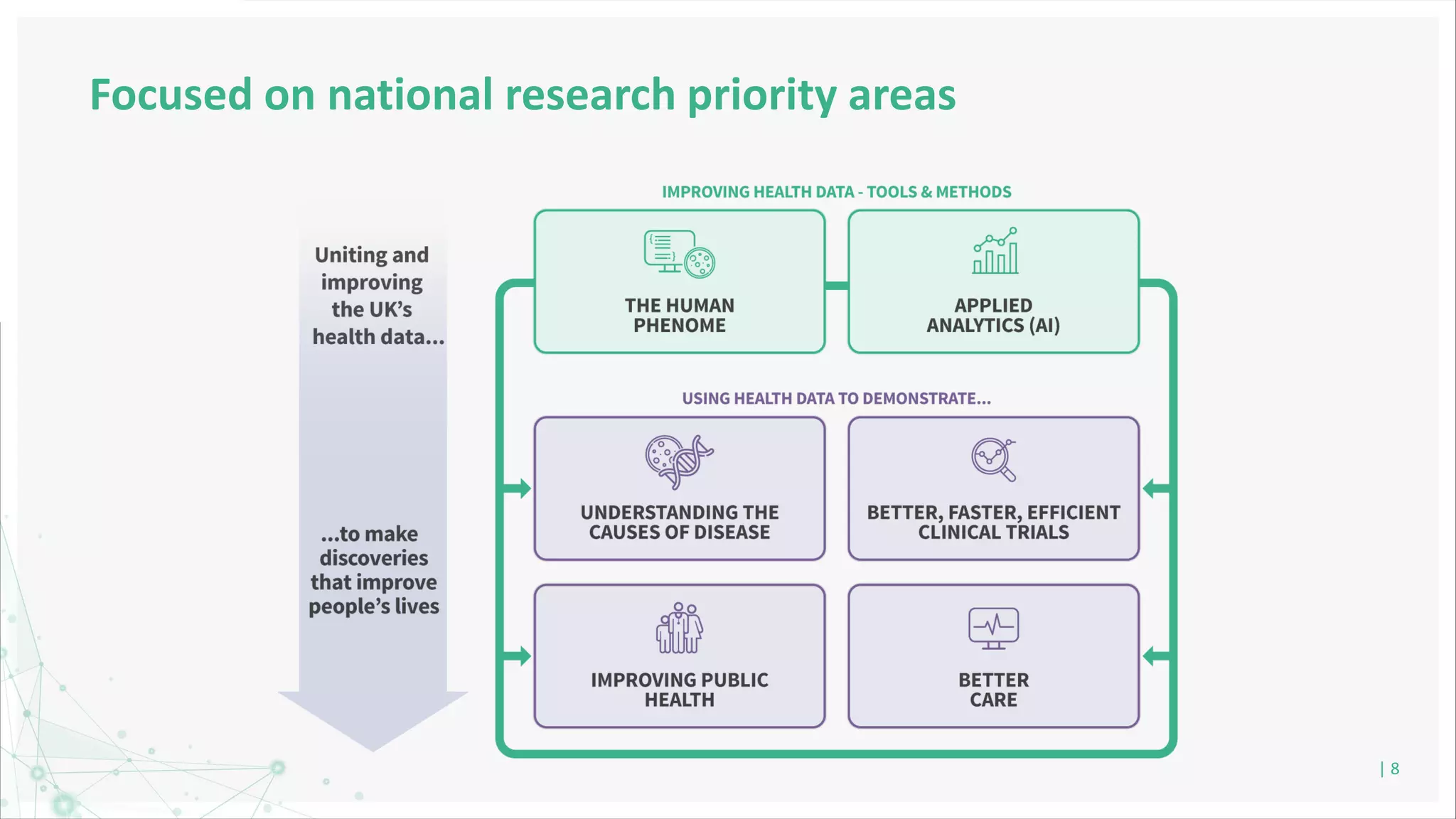
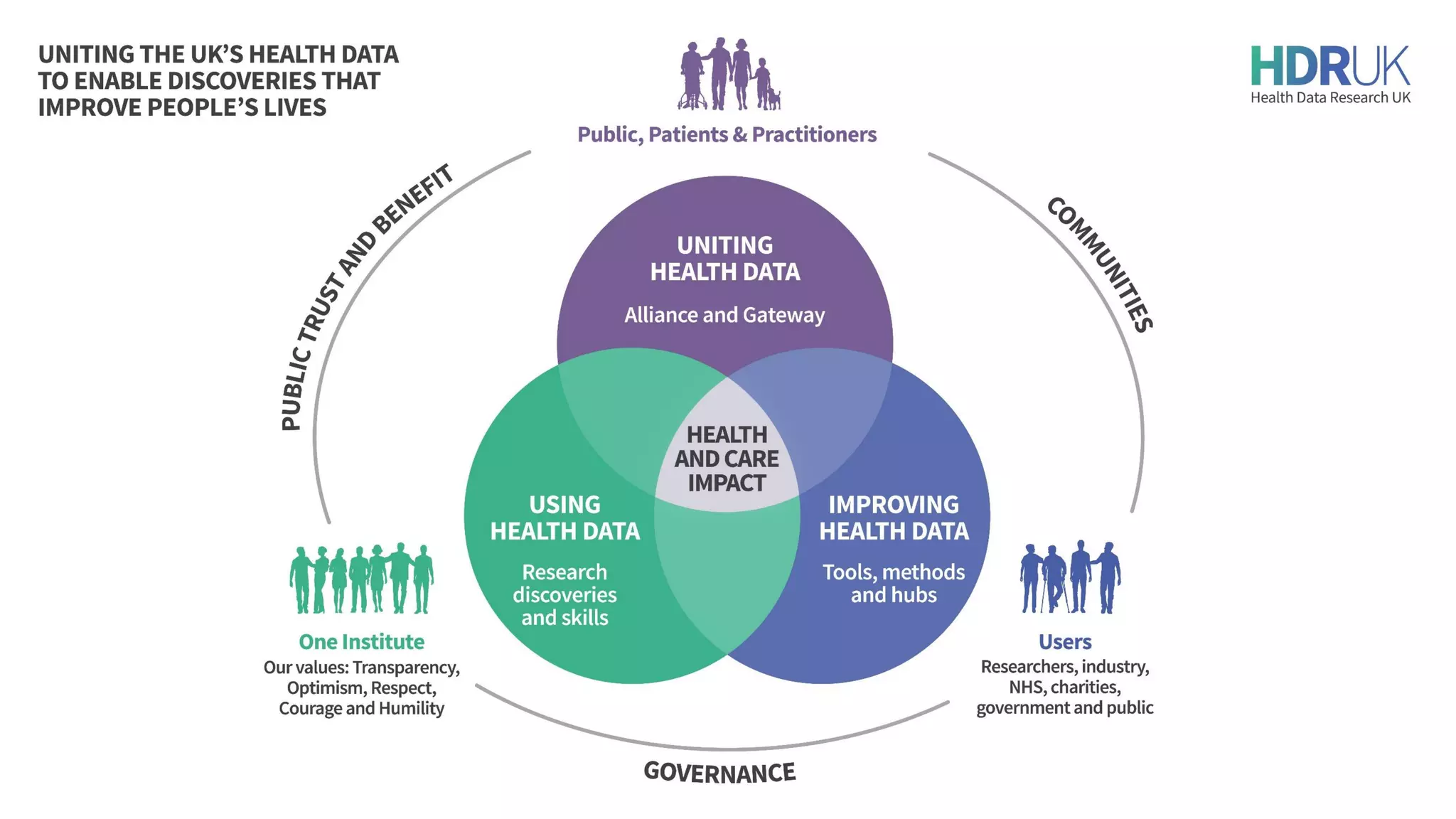
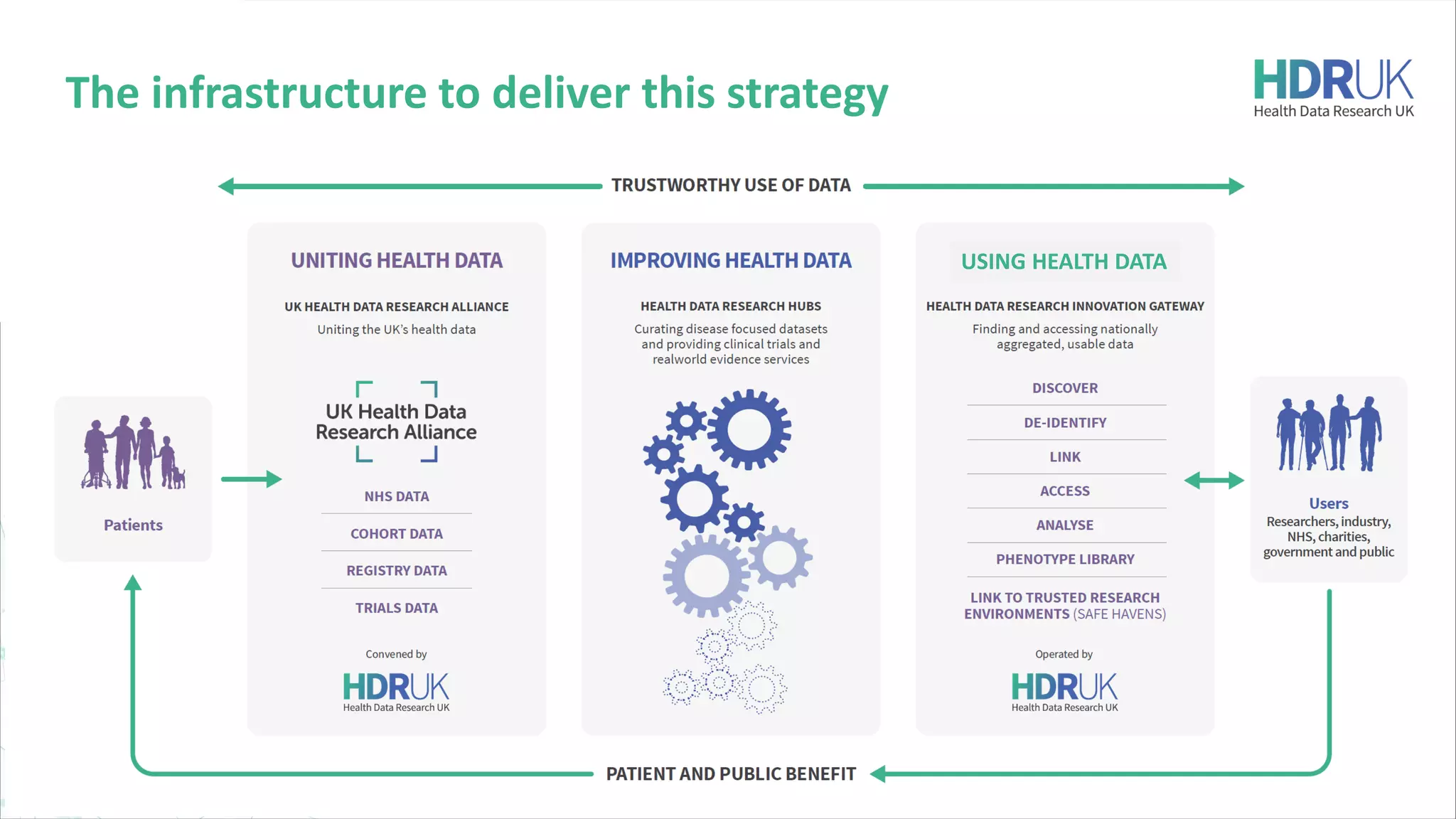
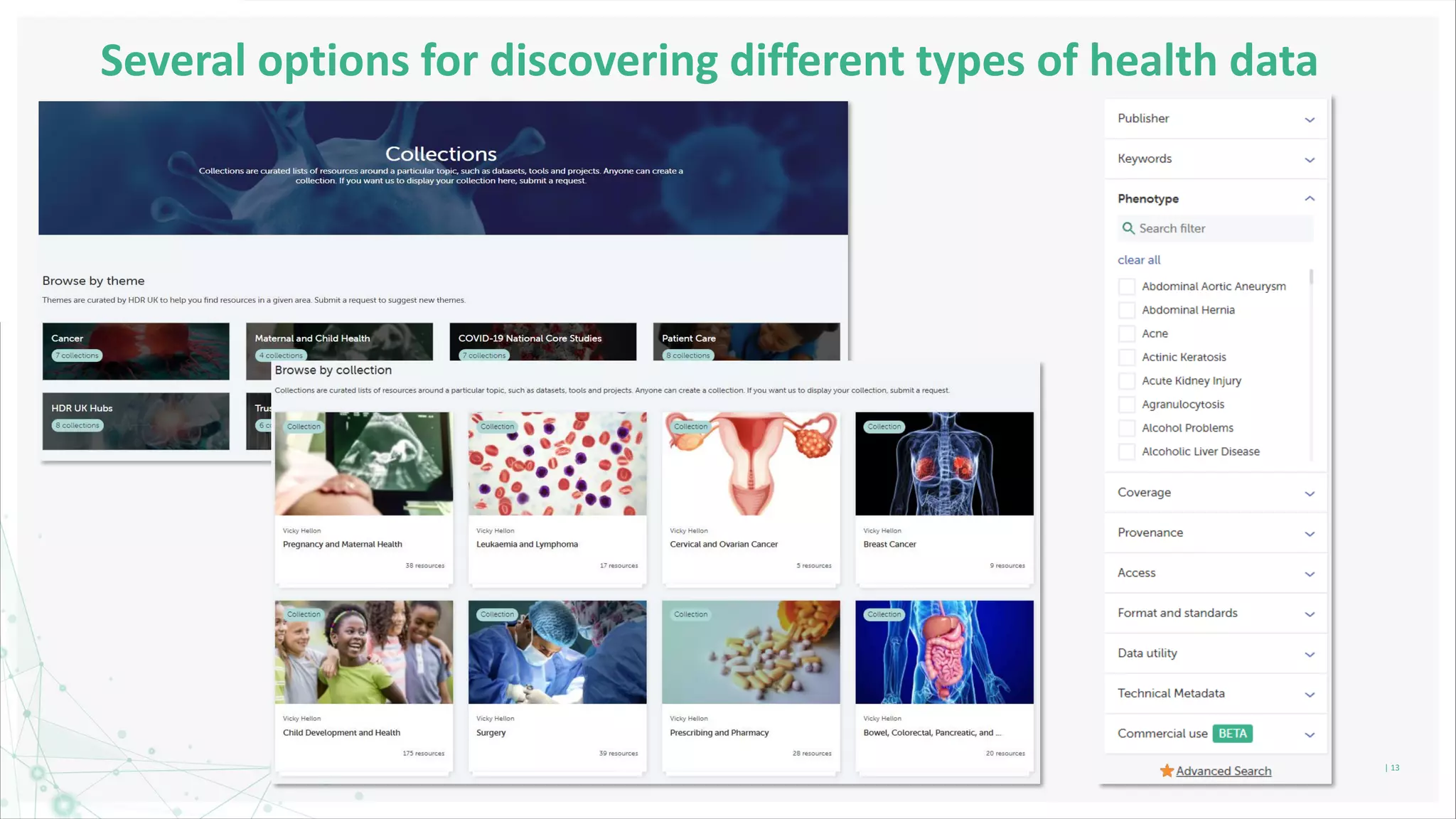
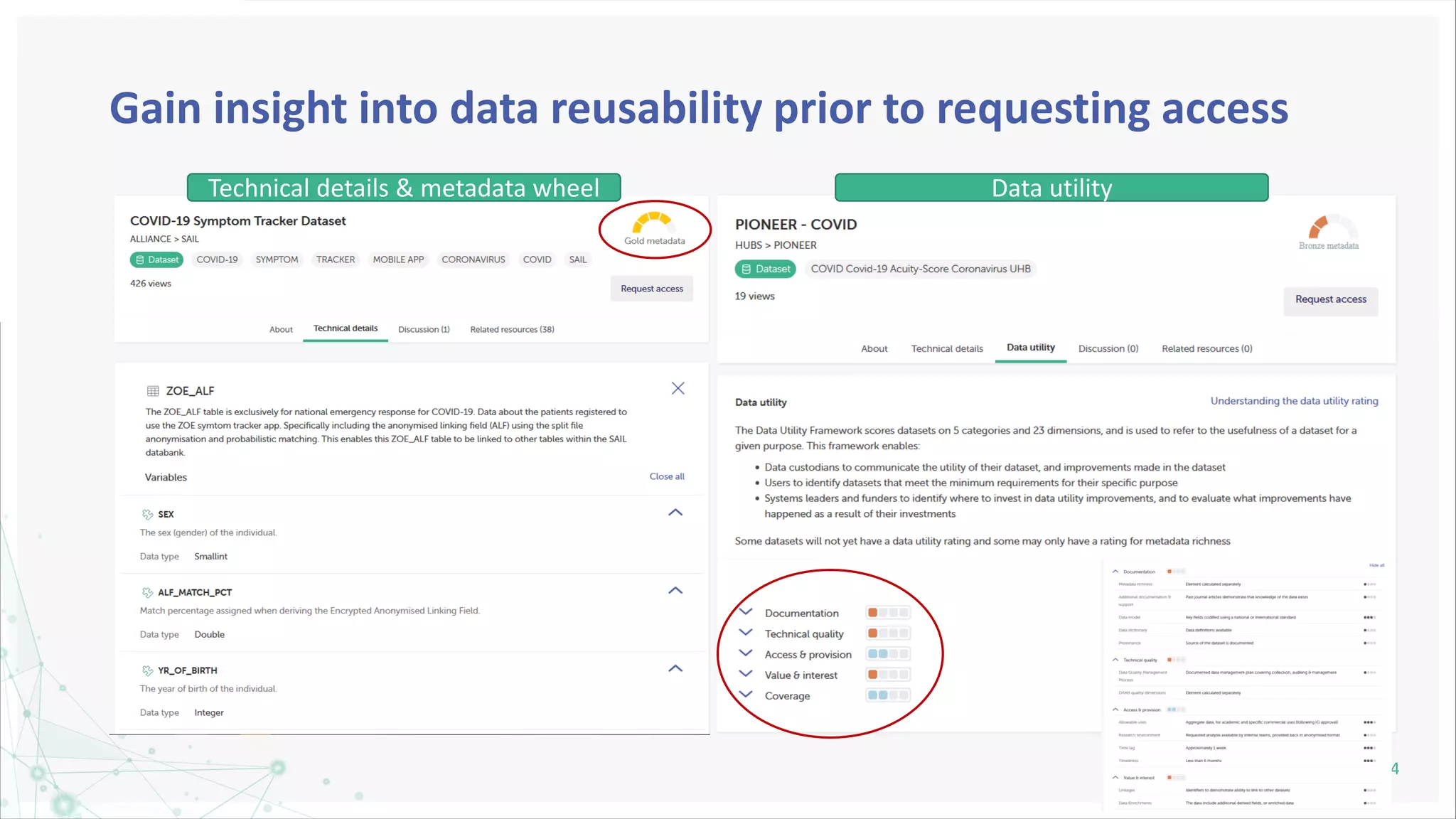
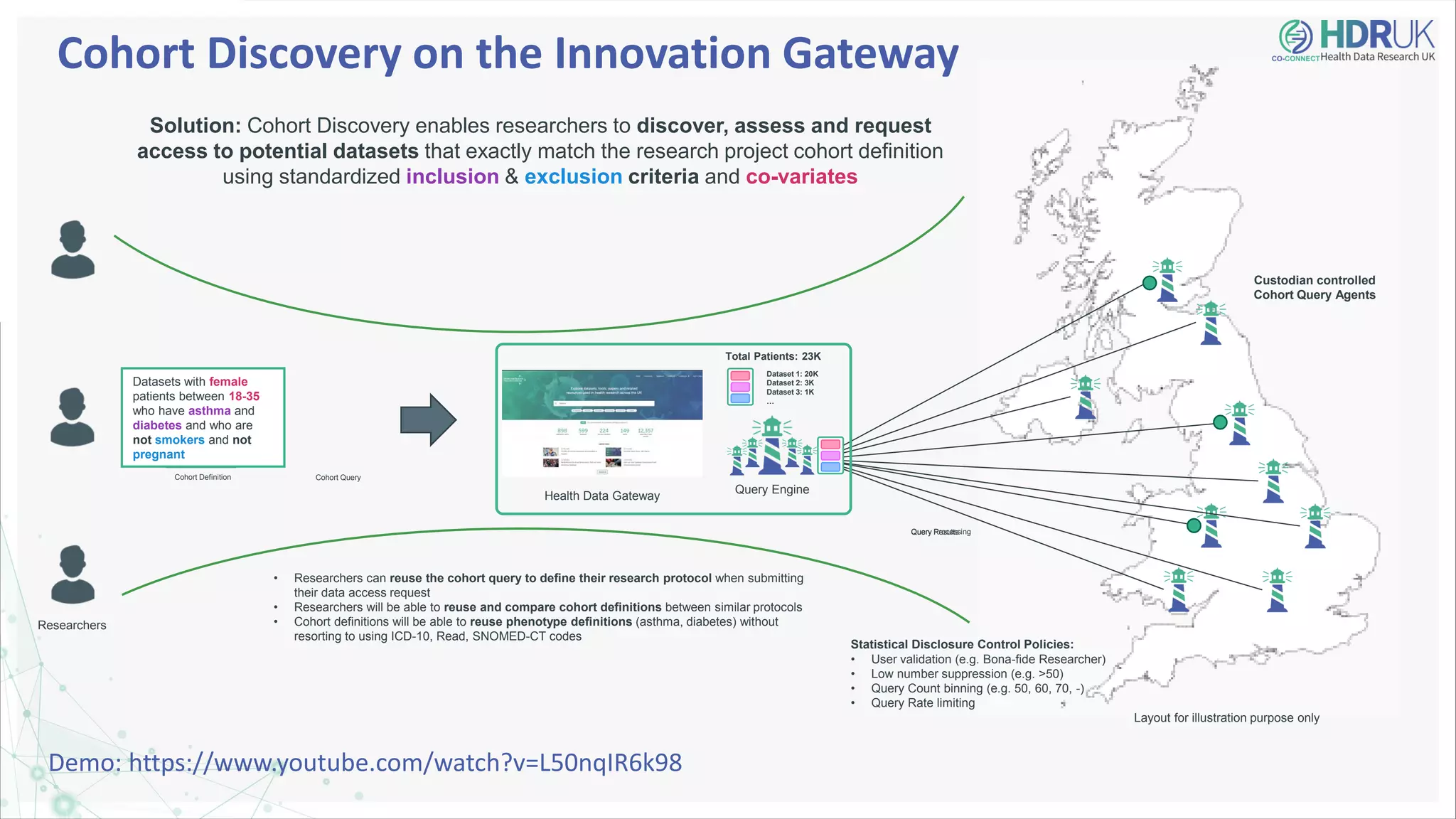
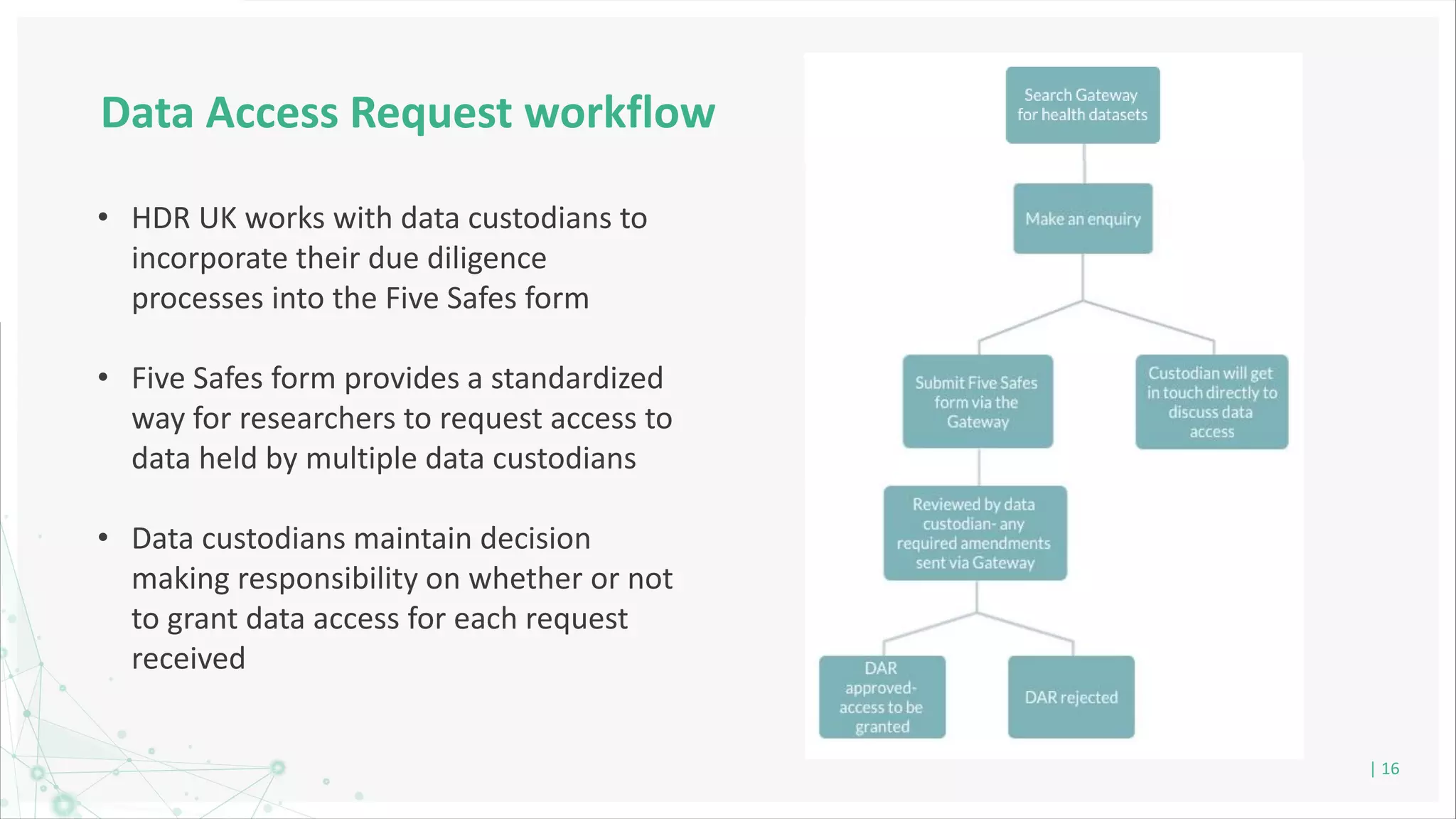
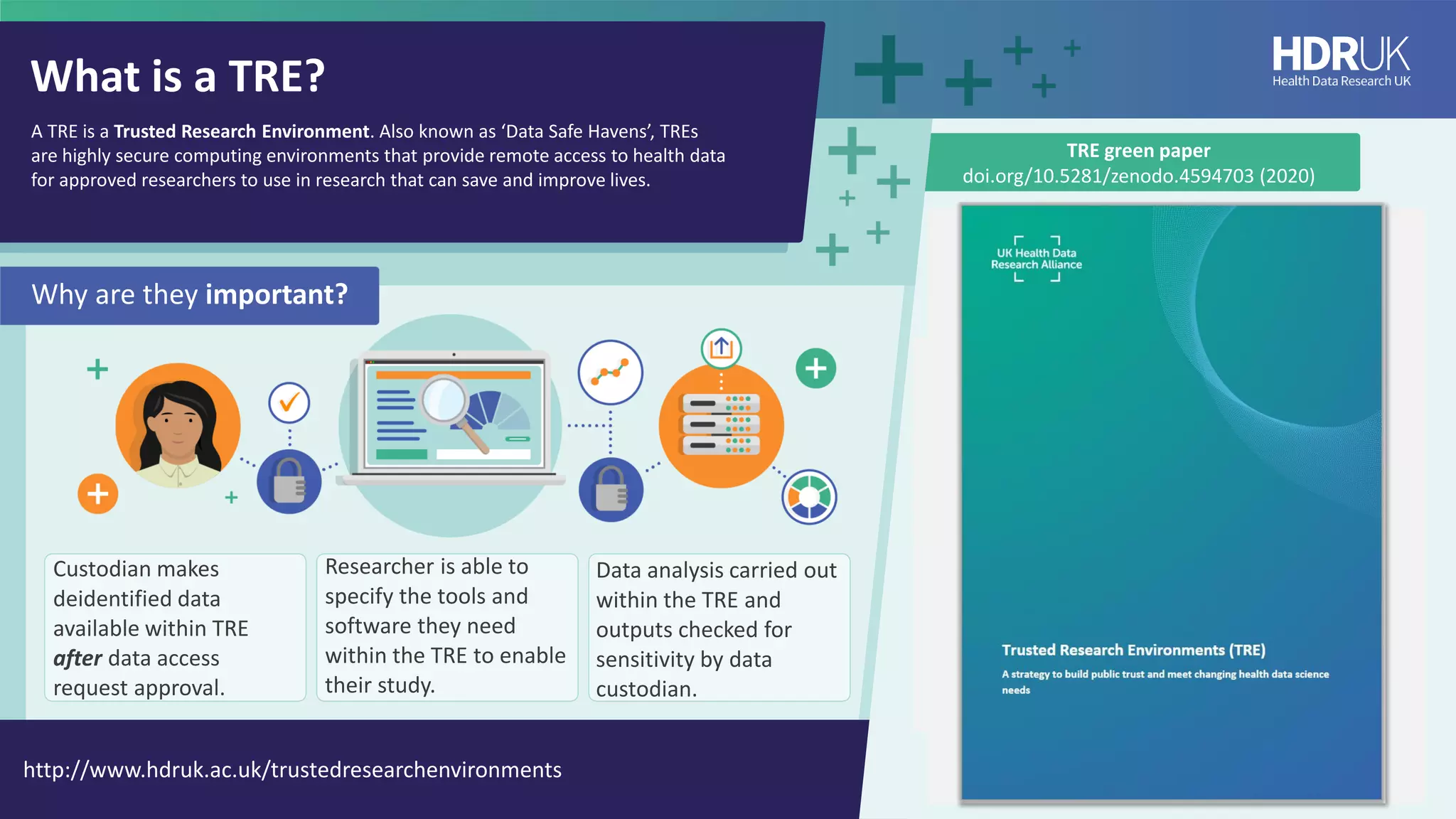
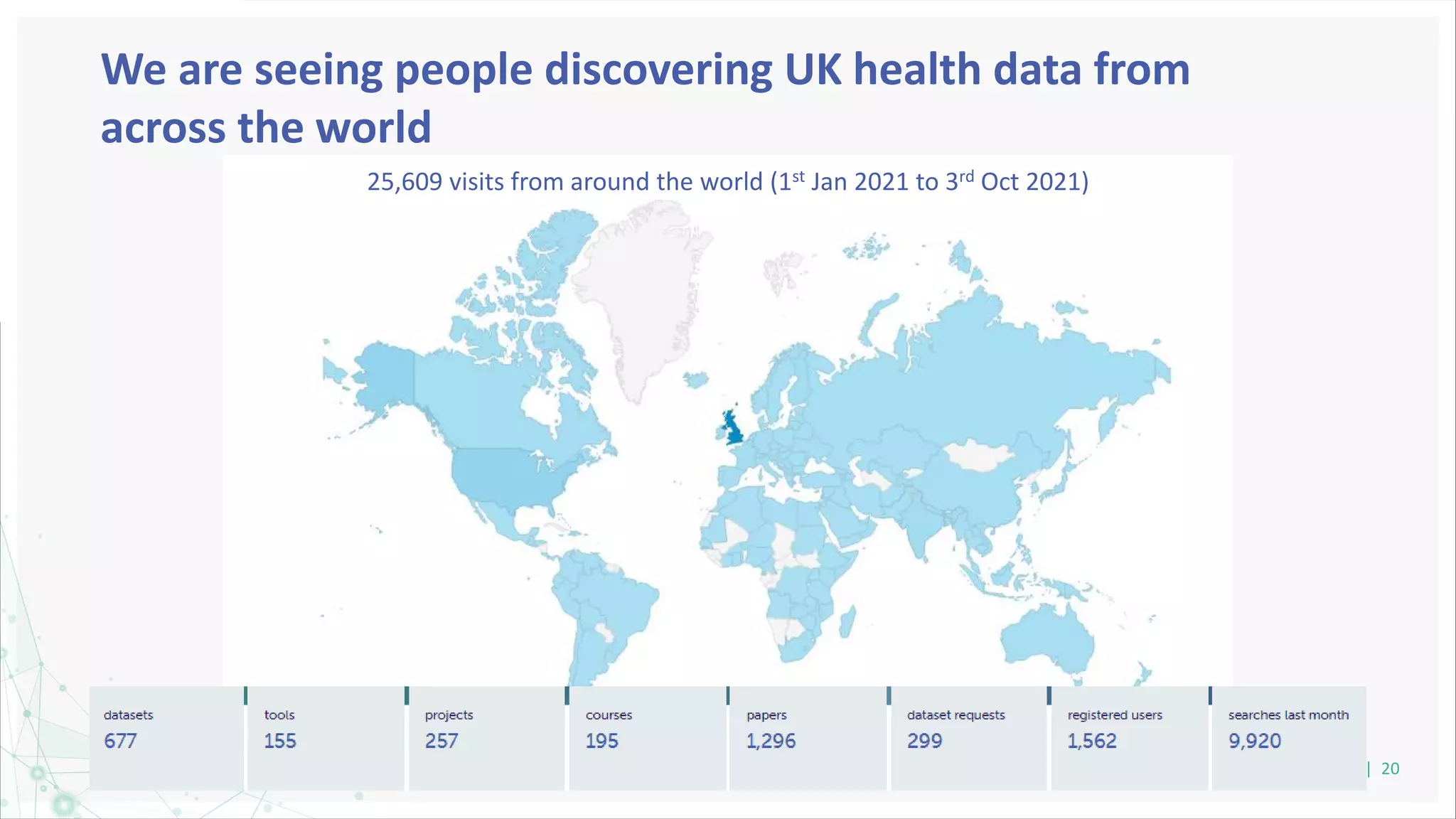
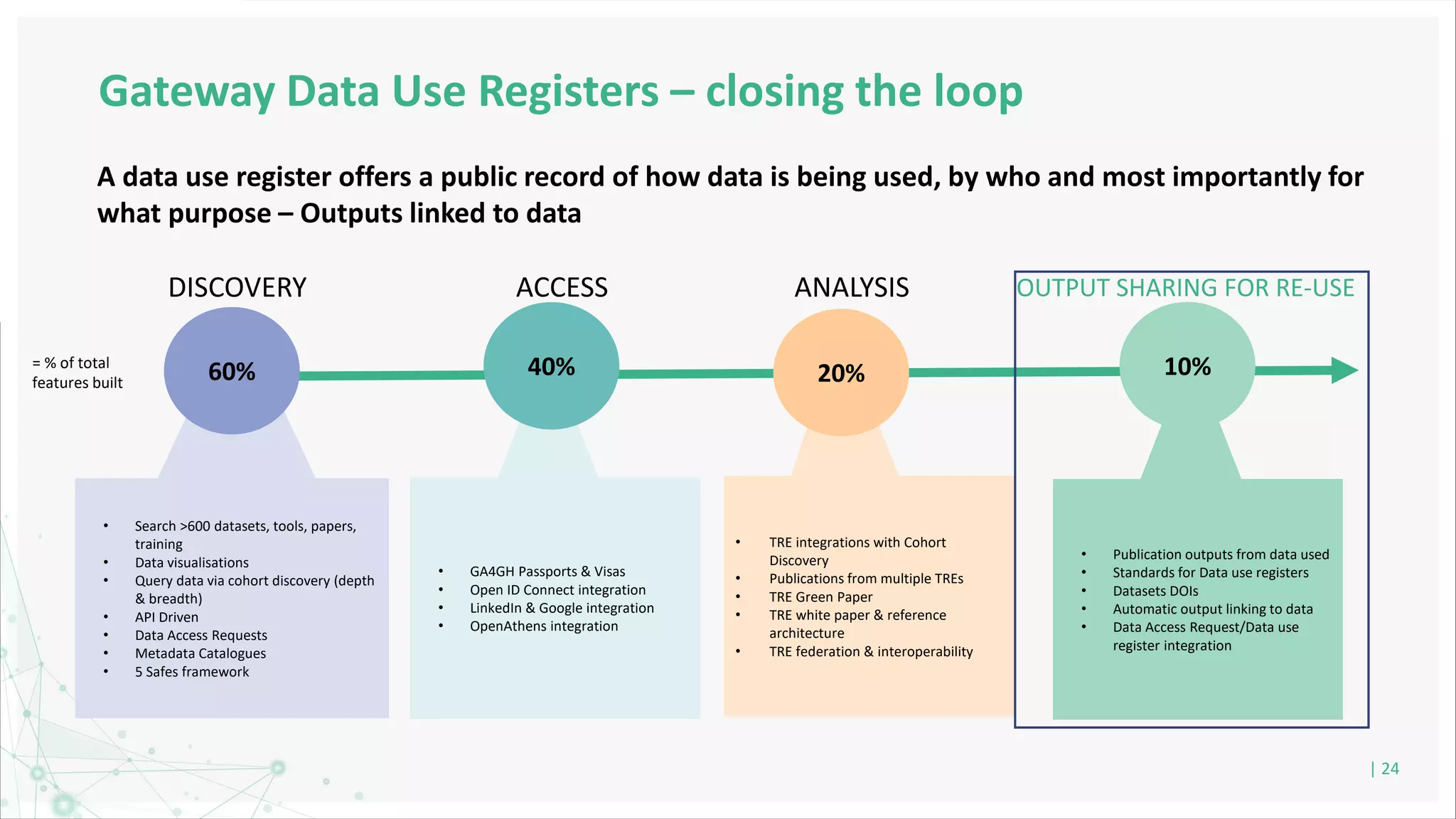
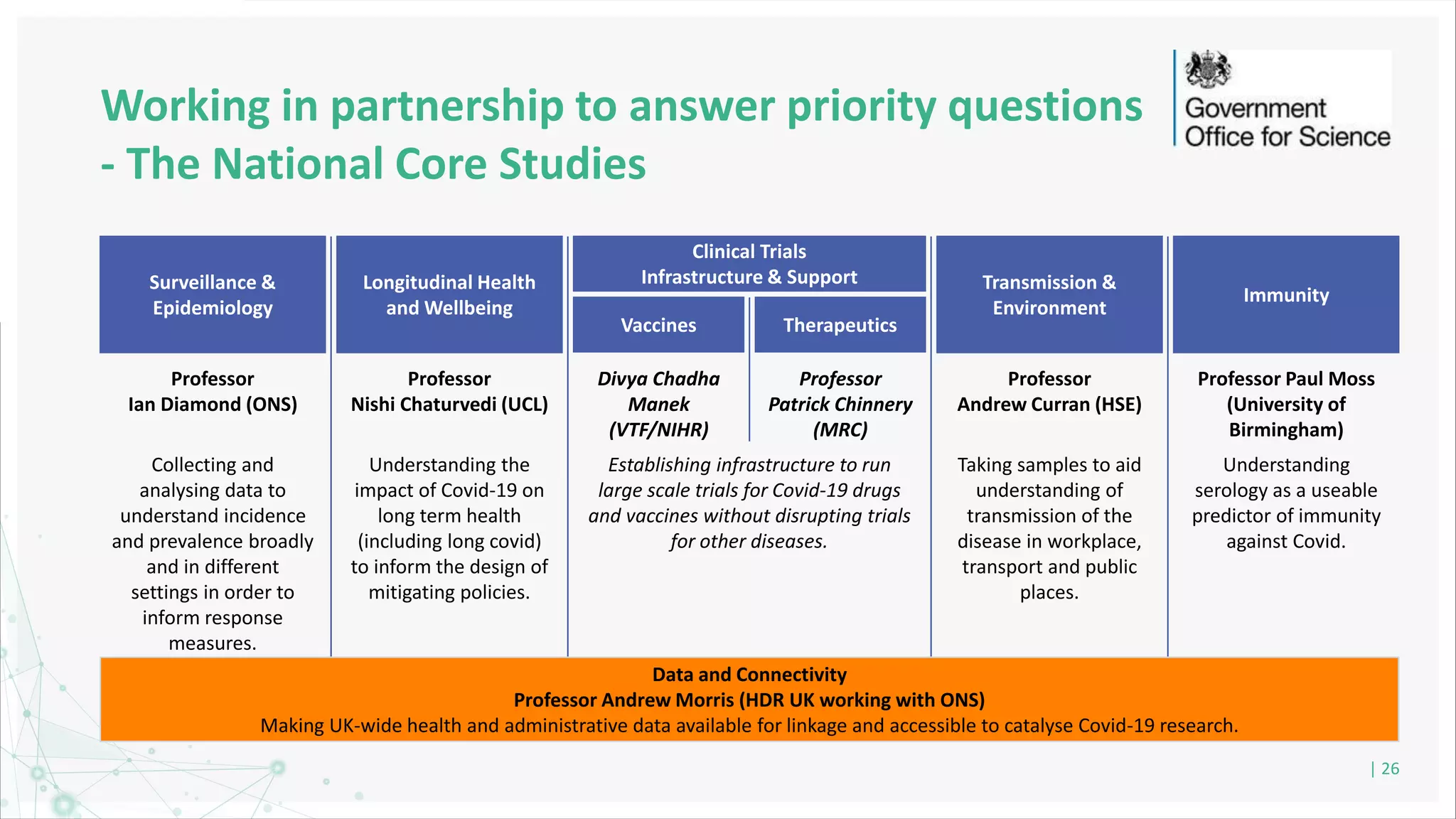
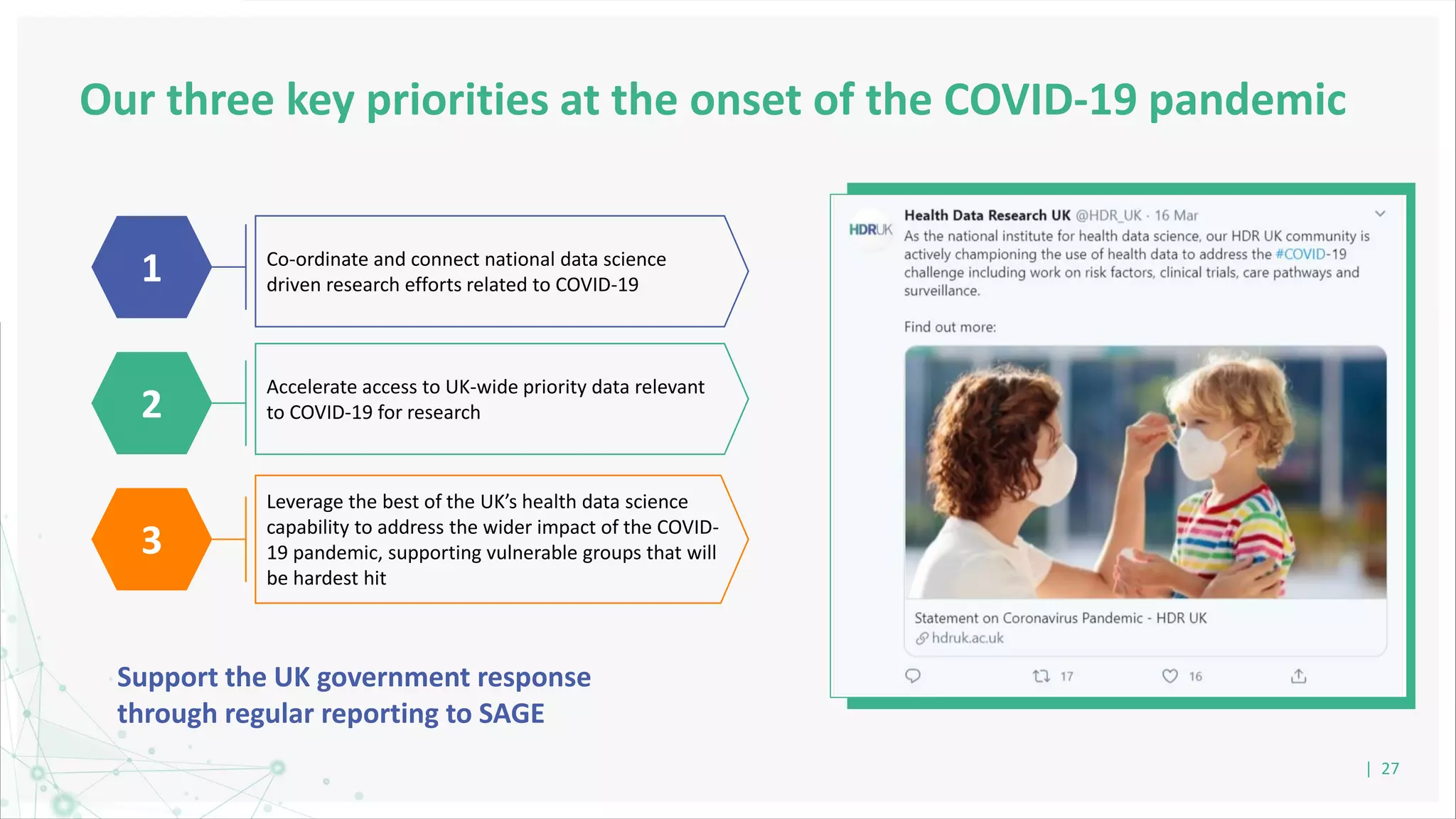
HDR UK is facilitating health data access in the UK for researchers through The Innovation Gateway. This allows researchers to discover and access de-identified health data from various custodians. HDR UK has emphasized transparency and patient/public involvement. During the COVID-19 pandemic, HDR UK coordinated data-driven research efforts and accelerated data access to support priority studies. This included enabling a clinical trial to more rapidly recruit participants using daily COVID test results. HDR UK is also laying the foundations for an international health data alliance to support open COVID-19 research globally.