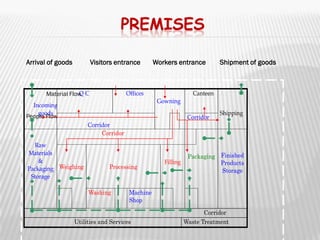
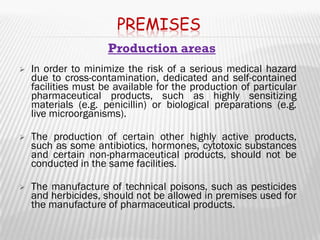
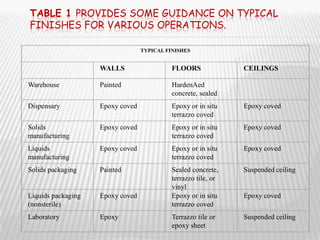
The document provides guidelines for GMP compliance related to premises, buildings, and facilities used for pharmaceutical manufacturing. It addresses requirements for design, construction, and maintenance of premises to minimize risks of contamination and ensure cleaning and maintenance. Specific areas covered include storage, production, quality control, and ancillary facilities. It also provides guidance on air handling, plumbing, lighting, sanitation, and other infrastructure.