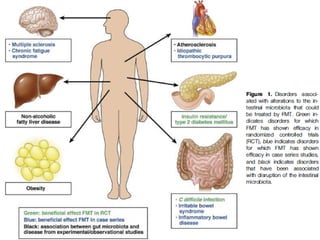
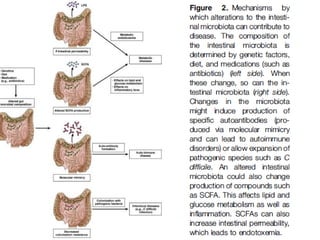
The document discusses the therapeutic potential of fecal microbiota transplantation (FMT). It describes how the gut microbiome can be altered by factors like diet, prebiotics, probiotics, and antibiotics. FMT has been shown to effectively treat recurrent or severe Clostridium difficile infection by restoring a healthy microbiome. The document reviews the methods of administering FMT and discusses its potential applications for other conditions like inflammatory bowel disease, metabolic disorders, and more. Controlled studies are still needed to fully evaluate FMT's therapeutic effects.