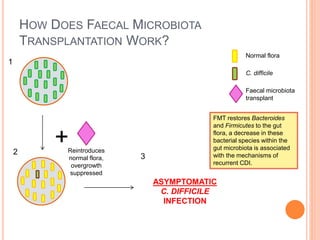
Faecal microbiota transplantation (FMT) involves transplanting stool from a healthy donor into a patient with recurrent Clostridium difficile infection (CDI) in order to restore the normal gut flora. FMT has a cure rate over 90% for recurrent CDI and works by reintroducing bacteria that can suppress C. difficile growth and prevent toxin production. While it has significant advantages over antibiotic treatment, standardization of procedures and screening donors is needed before FMT can become a first-line treatment option.