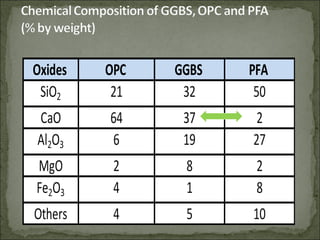
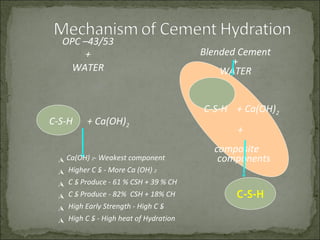
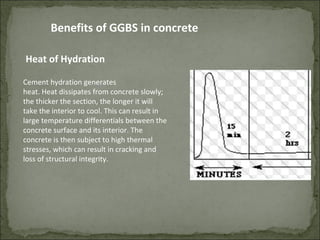
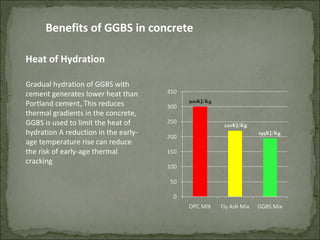
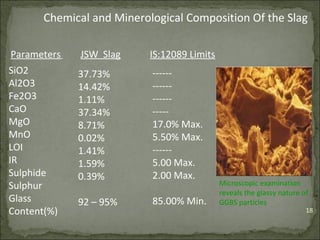
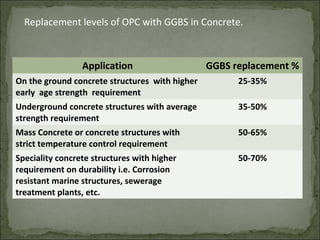
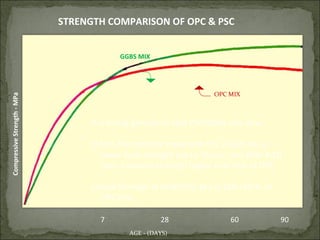
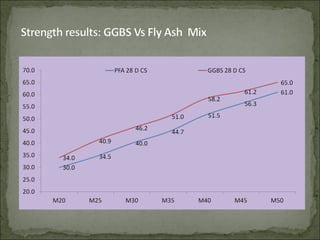
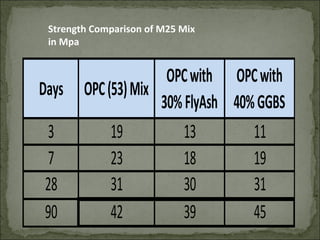
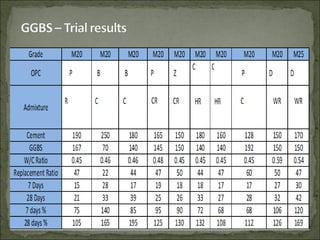
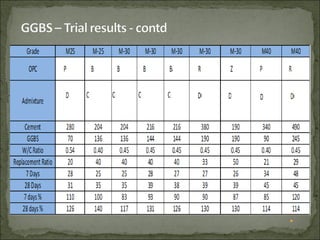
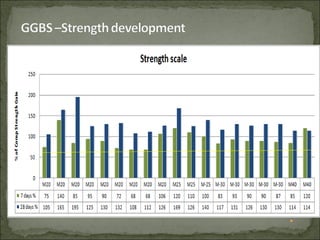
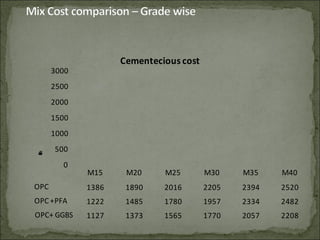
The document discusses the properties and benefits of using ground granulated blast furnace slag (GGBS) as a partial cement replacement in concrete. It states that GGBS has a similar chemical composition to Portland cement and when used in concrete it can reduce heat of hydration, water demand, and improve sulfate resistance, durability, and strength long-term. It recommends replacement levels of 25-70% GGBS depending on the application to optimize properties like workability, strength development, and cracking resistance.