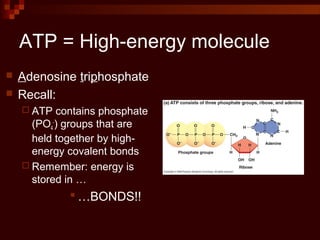
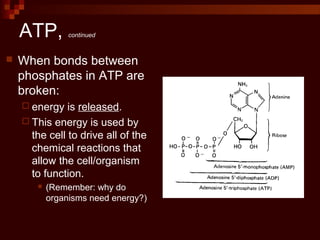
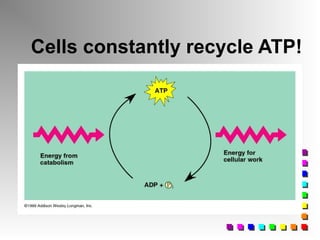
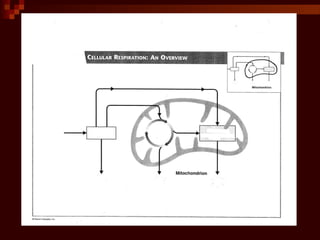
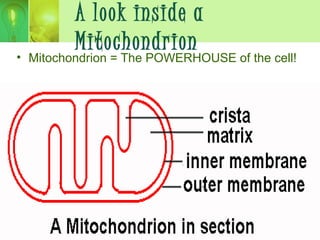
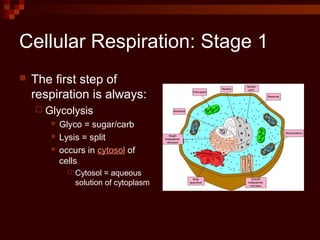
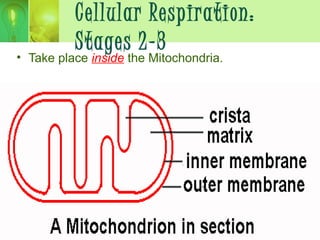
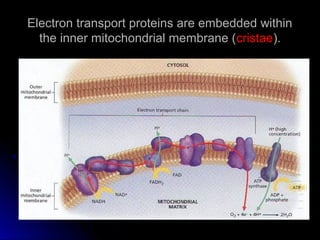
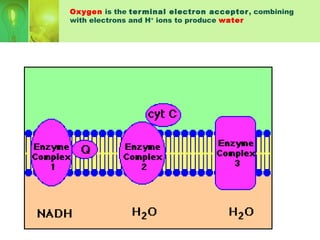
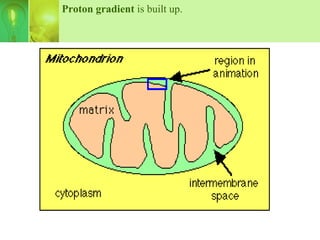
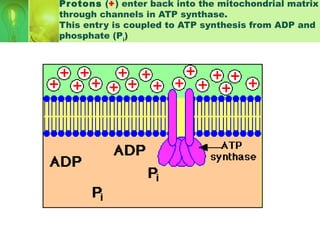
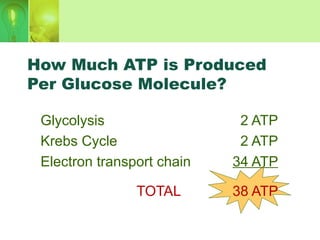
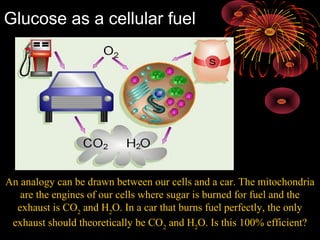
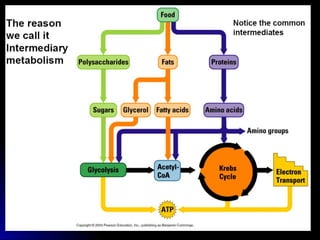
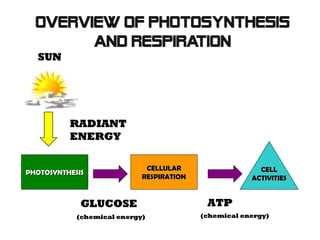
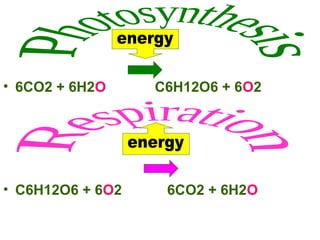
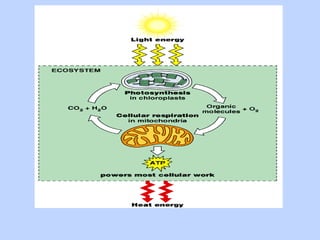
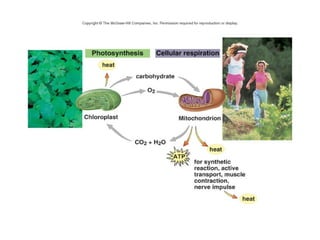
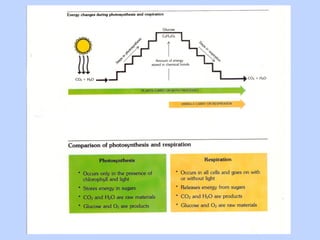
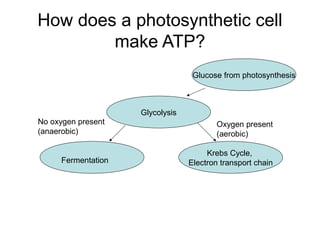
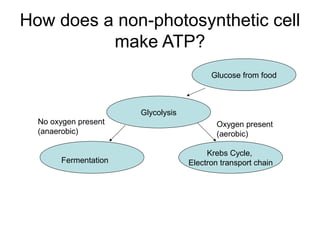
This document provides an overview of cellular respiration by discussing its three main stages: glycolysis, the Krebs cycle, and the electron transport chain. It explains that cellular respiration occurs in the mitochondria and releases energy from glucose and other food molecules to produce ATP, the energy currency of cells. The document also compares aerobic and anaerobic respiration, and contrasts how photosynthetic and non-photosynthetic cells generate ATP through these metabolic pathways.