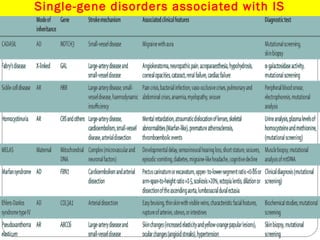
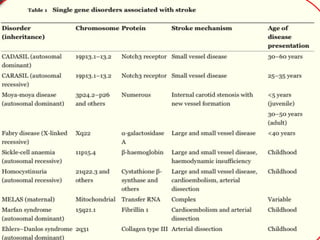
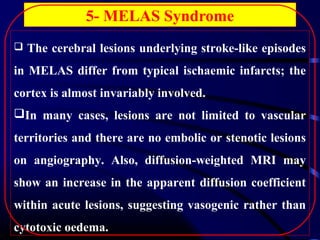
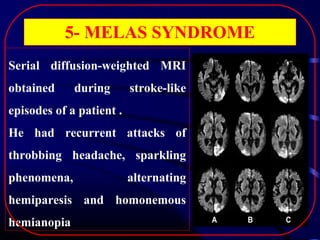
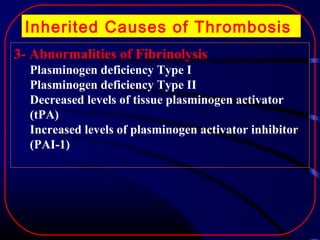
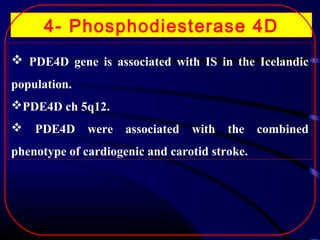
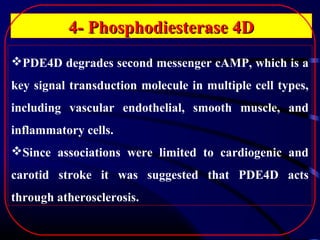
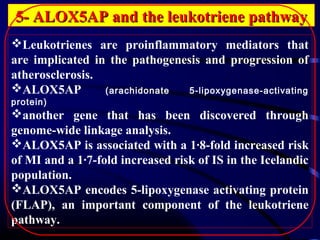
The document discusses several single-gene disorders that can cause ischemic stroke, including:
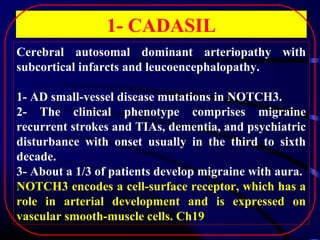
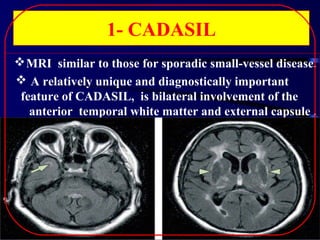
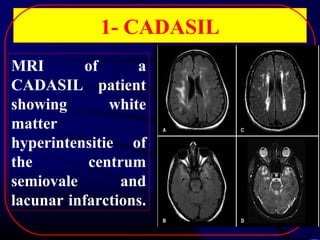
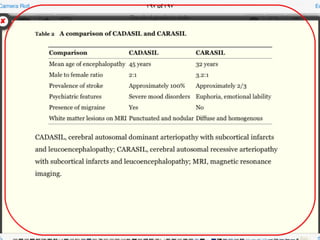
1. CADASIL, caused by mutations in the NOTCH3 gene, which is characterized by recurrent strokes, dementia, and MRI findings of white matter lesions.
2. Fabry's disease, an X-linked disorder caused by alpha-galactosidase A deficiency, which can cause both large and small vessel strokes in young patients.
3. Sickle cell disease, where strokes typically occur in childhood and are caused by intimal thickening leading to thrombus formation in large arteries or recurrent small subcortical infarcts.
It also briefly mentions other disorders like homocystinuria,