This document provides information on various chemistry concepts including:
1) The scientific method process of observation, hypothesis, experiment, and conclusion.
2) Differences between physical and chemical changes and examples of each.
3) Density concepts including the density formula and how density relates to floating and sinking.
4) Classification of matter including elements, compounds, mixtures, and various types of mixtures.













![Atom
Protons: + [1amu]
Electrons: - [0amu]
Neutrons: neutral [1amu]
Atomic mass: P +N
Atomic Weight: Weighted
average of isotopes
Isotopes: elements vary in neutrons
Ions: elements vary in electrons](https://image.slidesharecdn.com/generaloverview-160411143928/85/General-overview-15-320.jpg)

![Periodic Table: How to Read
Column: Group
Row: Period
Alkali Metals [group 1]
Alkaline Earth Metals [group 2]
Transition Metals [group 3-12]
Halogens [Group 17]
Noble gases [Group 18]
Metals shiny, good conductors, ductile
Nonmetals NOT
Metalloids Semiconductors](https://image.slidesharecdn.com/generaloverview-160411143928/85/General-overview-17-320.jpg)




![Classifying Type of Attractions
Ionic Bonding
Metal + Nonmetal [Greater than 1.8]
Hydrogen Bonding
FON
Dipole- Dipole
Polar [0.5-1.8]
Dispersian
Temporary Dipole Nonpolar [0-0.4]
1. Increase in melting point (ºC)
2. Increase in force of attraction](https://image.slidesharecdn.com/generaloverview-160411143928/85/General-overview-23-320.jpg)



![Atmospheric Pressure
Atmospheric pressure increase at lower
altitudes
[Less air inside chip bag]
Pressure in Bag < Atmospheric pressure
Atmospheric pressure decrease at higher
altitudes
[more air inside chip bag]
Pressure in Bag > Atmospheric pressure](https://image.slidesharecdn.com/generaloverview-160411143928/85/General-overview-29-320.jpg)







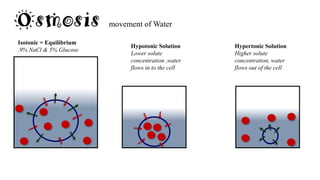
![solutions
Solutions
Homogenous
Solution
[transparent]
Goes through
filters & semi-
permeable
membranes
Colloids
Do not separate
or settle
Pass through
filters NOT
semipermeable
membrane
Suspensions
Seen by naked Eye
Trapped by filters
& semipermeable
membrane
[Heterogeneous]](https://image.slidesharecdn.com/generaloverview-160411143928/85/General-overview-38-320.jpg)

![Unsaturated
[NOT full capacity]
Saturated
[Full capacity]
Solute dissolves
Solute Recrystallizes
Solvent + Solute Saturated Solution](https://image.slidesharecdn.com/generaloverview-160411143928/85/General-overview-40-320.jpg)
![Solubility Rules
PO4
3-
Co3
2-
S2-
NH4
+
Na+
K+
NO3
-
Li+
All Soluble
C2H3O2
-
Cl-
Br-
I-
SO4
2- OH-
All Soluble
Except
Iron (III) Acetate
Fe(CaH3O2)3
All Soluble
Except
Ag+, Pb2+, Hg+
PbCl2, PbBr2 [slightly]
All Soluble
Except
Ba2+, Pb2+, Ca2+,
Sr2+, CO32-, S2+,
PO43-, OH-, Ag+
Only Alkali
Metals
& NH4+
Only Alkali Metals
& NH4+
Ca2+, Ba2+, Sr2+ [slightly]](https://image.slidesharecdn.com/generaloverview-160411143928/85/General-overview-41-320.jpg)






![[H3O+] > [OH-] Acidic
[H3O+] = [OH-] neutral
[H3O+] < [OH-] Basic
How to define pH](https://image.slidesharecdn.com/generaloverview-160411143928/85/General-overview-48-320.jpg)
![7 More [Basic]Less [Acidic]
Calculation:
[H3O+]=10-ph
1) Whole number Plug in as pH
2) NOT whole number & switch to negative 10(-ph)
pH=-log[H3O+]
1) Plug in H3O+ number
2) -log (#)
3) Make sure setting scientific notation](https://image.slidesharecdn.com/generaloverview-160411143928/85/General-overview-49-320.jpg)


