The document provides an overview of the National Institute of Open Schooling (NIOS) and the educational services offered by educator Manish Verma, which include guidance for students through a YouTube channel. It discusses various concepts in chemistry, including atomic structure, chemical combinations, and reaction calculations, aimed at helping students in their studies. Additionally, it underlines the importance of NIOS in providing accessible education since its establishment in 1989.



![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
3 | P a g e CHEMISTRY (313)
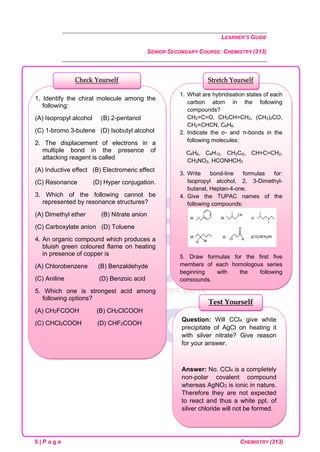
1. C-O bond length is minimum in
(A) CO2 (B) CO3
2-
(C) HCOO–
(D) CO
2. Molecules are held together in a crystal
by
(A) Hydrogen bond
(B) Electrostatic attraction
(C) Van der Waal’s attraction
(D) Dipole-dipole attraction
3. Sp3
d2
hybridization is present in
[Co (NH3)6
3+
], find its geometry
(A) Octahedral geometry
(B) Square planar geometry
(C) Tetragonal geometry
(D) Tetrahedral geometry
4. Find the molecule with the maximum
dipole moment
(A) CH4 (B) NH3
(C) CO2 (D) NF3
5. MX6 is a molecule with octahedral
geometry. How many X – M – X bonds are
at 180°?
(A) Four (B) two
(C) Three (D) Six
Check Yourself
1. In SF4 molecule, the lp electrons
occupies an equatorial position in the
trigonal bipyramidal arrangement to an
axial position. Give reason.
2. Write electron dot structure (Lewis
structure) of Na, Ca, B, Br, Xe, As, Ge,
N3-
. Out of p-orbital and sp-hybrid orbital
which has greater directional character
and Why?
3. Explain the shape of BrF5.
4. Explain why PCl5 is trigonal bipyramidal
whereas IF5 is square pyramidal.
5. In both water and dimethyl ether
(CH3— Ö — CH3), oxygen atom is central
atom, and has the same hybridization, yet
they have different bond angles.
Stretch Yourself](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-4-320.jpg)


![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
1 | P a g e CHEMISTRY (313)
• Atom (Given by Dalton): Matter is
made up of extremely small particles
which are indivisible in nature. It consists
of subatomic particles electron, proton
and neutrons knows as fundamental
particles.
1. Electron (Named by Stoney):
Discovered by Cathode Ray experiment
[In crook’s tubes]. A long glass tube
with two metal electrodes. At every low
pressure when high voltage is applied a
flow is produced due to flow of − ve
charge particle [known as electron],
cathode rays. Cathode rays have −ve
change, travel in straight lines has
electric and magnetic field have heating
effect more penetrating effect. Charge
on e- was found by Oil drop experiment
[Millikan].
2. Proton (Discovered by Goldstein in
anode ray experiment: In a perforated
cathode tube with gas at low pressure
high voltage was passed between
electrode rays from cathode produced
green fluorescence on ZnS all. These
were called as anode rays. They travel
in straight line, with + ve charge, get
defected in electric and magnetic field.
3. Neutron: Fundamental particle which
carries no charge but has mass equal to
N atom or Proton. Discovered by James
Chadwick.
Table 2.1 Fundamental particles of atom and their
characteristics
Atomic Number, Mass Number,
Isotopes and Isobars
• Atomic number (Z): The no. of protons or
electron in a neutral atom or No. of protons
in an atom (or ion).
• At mass no. (A): Total no. of protons and
neutron in an atom
• Isotopes: Atoms of same element with
different mass no.
• Isobars: Atoms of different element with
same mass no.
• Isotones: Atoms of different element with
same no. of neutron.
• Isoelectronic: Atoms, molecules or ions
with same no. of e-[Ne; O2-].
Earlier Models
Thomson’s Model
• J.J. Thomson: The sphere of +ve change
nucleus model of atom is 14 Chemistry
balanced by coulombic force of attraction
of e-. Like a Raisin Pudding Model
Fig. 2.1: A pictorial representation of Thomson’s plum-
pudding model
ATOMIC STRUCTURE
2](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-7-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
2 | P a g e CHEMISTRY (313)
Rutherford’s Experiment
• Ruther Ford (Discovery of nucleus):
particles (+ve charge) bombarded on
gold foil.
a. 99.9% passed without deflection:
Most space inside the atom is empty.
(b) Only few deflected therefore mass
of atom centrally placed called
nucleus.
b. Very few deflected back therefore mass
of atom contains +ve charge particles
[Protons].
c. Atom is electrically neutral hence −ve
change particles placed outside the
nucleus and have very less mass.
• Limitations: No distribution and
energies of e-considered, could not
explain e- does not fall into the nucleus
or not; no details of line spectra of H
atom.
Electromagnetic Radiations
• Energy emitted from any source (in
forms of waves) in which electric and
magnetic fields oscillated perpendicular
to each other and travelling with a
velocity to light is known as EM
radiation.
Characteristic Parameters of
Electromagnetic Radiations
a. Wavelength: the distance of one crest
and one trough in a wave. Denoted by ‘’
b. Frequency:no. of waves
passing through a given point in one
second.
c. Amplitude: The height of crest or depth
of a trough denoted by ‘a’
d. Wave no.: No. of waves per unit length
denoted by
e. Velocity: Linear distance travelled by a
wave in one second.
Electromagnetic Spectrum
• Energy wise order for EM radiation.
Line Spectrum
• When the vapors of some volatile
substance are allowed to fall on the flame
of a Bunsen burner and then analyzed
with the help of a spectroscope. Some
specific-colored lines appear on the
photographic plate which is different for
different substances. For example, sodium
or its salts emit yellow light while
potassium or its salts give out violet light.
Line Spectrum of Hydrogen Atom
• Hydrogen spectrum: When e- in
hydrogen atom is provided energy it
gets excited to higher shell from ground
state, it comes back to ground state by
emitting energy in definite values.
• “Quanta”: The emission of light energy
is known as emission spectra. It
corresponds to each atom depending
upon which energy shell e-is excited. It
is discontinuousspectra as ‘’ of light](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-8-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
3 | P a g e CHEMISTRY (313)
radiations do not merge with each other
like is VIBGYOR (Continuous Spectra).
When e- falls from any excited state to
a. Ist energy level nf = 1, ni= 2, 3, 4, ....
[Lyman series] (UV)
b. When e- to final state nf = 2, ni= 3, 4,
5, .... [Balmer series] (VIBGYOR)
c. When e- to falls to final state nf = 3
ni = 4, 5, 6 [Paschem series] IR.
d. When e- to falls to final state nf =4 ni
= 5, 6, 7 [Bracket series] IR.
e. When e- to falls to final state nf =5ni
= 6, 7, 8[Pfund series] IR.
Bohr’s Model
• Bohr’s theory for H [H like one e-
systems He+; Li2+]e-revolving round
the nucleus in circular path [stationery
state; [Shell]With a definite angular
momentum [n no. of shell of e-]
and with definite energy
• As n increases Z Decreases Energy of
e- becomesless -ve [Due to less,
force of Proton attraction]
• As n Decreases Z increases Energy of
e- becomes More -ve [Due to more
force of attraction by protons]
• In infinity shell e- has zero force of
attraction therefore zero energy.
• Electron energy only changes by
definite values E = Ef −Ei .
Wave – Particle Duality
• Debroglie equation: All material particles
possess both matter should also exhibit
wave like properties. Wave character as
well as wave character as well as wave
character.
• For microscopic particles mass is very less
therefore ‘’ more and more wave
character.
• For macroscopic particles mass is large
is less therefore more particle character.
Heisenberg’s Uncertainty Principle
• It is impossible simultaneously to determine
the exact position and exact velocity of a
subatomic particle.
• For microscopic (mass very less) certainty
in position is less therefore x is more v is
less.
• For macroscopic (large mass) certainty in
position is more x is less v is more.
Wave Mechanical Model Of Atom
• Erwin Schrödinger proposed the quantum
mechanical model of the atom, which treats
electrons as matter waves. The wave
mechanical model proposed that the electrons
act like particles as well as waves of energy.
According to the fields around, the electrons
change their path and they move very fast,](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-9-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
4 | P a g e CHEMISTRY (313)
hence they are not in one place during
any particular time. The wave
mechanical model was used for the
construction of an atom.
Significance of Quantum
Numbers
1. Principal Q. No. : It describes the
distance of e- from nucleus ‘n’ i.e.,
defines the shell no. It is denoted by
‘n’.
2. Azimutha Q. No. : It defines the path
of e- decided by angular momentum of
e-. Each angular momentum value
corresponds to one subshell. The no.
of subshells in a shell is 0 to n−1.
All subshells are wave functions for
locating e-In the same shell energy
wise S < P < d < f
3. Magnetic Q. No. : It gives the no. of
magnetic orientations an e- can have
in a subshell. The no. of magnetic
orientation an e- can have in a
subshell.
4. Spin Q. No. : An e- is continuously
spinning on its own axis. This Q. No.
describes e- can have clockwise spin
motion or e- can have
anticlockwise spin motion . An
orbital can have mximum two e- one
with clockwise and other with
anticlockwise spin.
Electronic Configuration of
Elements
Aufbau (or building up) Principle
a. e- are filled in increasing order of energy of
subshell.
b. As ‘n + l’ value increases energy of e-
increases in that subshell.
c. For two subshells with some ‘n + l’ value.
As ‘n’ value increases energy of e-
increases.
Pauli’s Exclusion Principle
• No two e- can have same set of 4
quantum nos. If two e- are present in
same shell, subshell, orbital they will have
different spin value.
Hund’s Rule
• The pairing of e- in degenerate orbitals
(different orbitals with same energy) will
get paired only once they have been
singly occupied. The no. of [Spherical
nodes or radical nodes] = n - l -1.
Shapes of Orbitals
Fig. 2.18: The boundary surface diagrams (shapes) of the s, p,
d-orbitals
Difference between psi and psi square:
Difference between Orbit and Orbitals:](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-10-320.jpg)


![NIOS/Acad./2021/313/02/E
National Institute of Open Schooling
Senior Secondary Course: Chemistry
Chapter-2 (Atomic Structure)
Worksheet-2
1. What experimental evidence shows the dual nature of light?
(a) Calculate the energy of the FM radio signal transmitted at a frequency of 100 MHz.
(b) What is the energy of the red colored wave with 670 mm wavelength?
2. How is the Bohr model superior to the Rutherford model?
3. Wavelength to green light is 335 millimeters. Calculate the energy of green photons.
4. How did the wave mechanics model of Atom develop?
5. Calculate the wavelength corresponding to the balmar line n=3.
6. If a 380 gram cricket ball is thrown at a speed of 140 kilometers per hour, calculate the de
Broglie wavelength.
7. Describe the hunds rule of maximum multiplicity with five examples.
8. Which oxidation state is more stable and why?
(a) Fe2+
or Fe3+
(b) Mn2+
or Mn3+
(c) Electronic configuration of Cr is [Ar] 3d5
, 4s1
and not 3d4
, 4s2
9. Which of the following class has the first storage and why?
(a) 2p or 3s (b) 3d or 4p (c) 4S or 3d
10. What is the significance of the azimuthal magnetic and spinning quantum numbers?
(a) Write all the four quantum numbers for, 3p3
(3rd electron), 4d5
(4th electron), 6S2
(2nd
electron).
(b) How many electrons are s=+1/2 and ml=0 for n=4](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-13-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
1 | P a g e CHEMISTRY (313)
The first classification of elements was
provided by Russian chemist D.I.
Mendeleev
The physical and chemical properties of
elements are periodic functions of their
atomic weight.
Modern Periodic law: The physical and
chemical properties of elements are
periodic functions of their atomic
numbers. It is the long form of periodic
table:
Periods→Horizontal rows
Group→Vertical columns
The horizontal rows on the periodic
table are called periods. The periods are
numbered 1 through 7 on the left-hand
side of the table. Elements that are in the
same period have chemical properties
that are not all that similar.
Period: There are total seven periods 1st
period 2 elements 2nd and 3rd period 8
elements 4th and 5th period 18 elements
6th and 7th period period 32 elements.
The vertical columns on the periodic
table are called groups or families
because of their similar chemical
behavior. All the members of a family of
elements have the same number of
valence electrons and similar chemical
properties.
Group: There are total eighteen Groups.
Groups 1 and 2 ‘s’ block elements last
electron entered in ‘s’ subshell [s1, s2] 3 to
12 ‘d’ block elements last electrons
entered in ‘d’ subshell [d1 to d10]. 13 to 18
‘p’ block elements last electrons enter in
‘p’ subshell [p1 to p6]. Group 18 Noble
gases.
(A) In ‘s’ and ‘p’ block elements the
electrons enters outer most shell. In ‘d’
block elements the electron enter the
penultimate shell (n-1). ‘f’ block elements
last electron enter the sub penultimate
shell (n-2).
(B) ‘f’ block elements are placed in
between ‘d’ block elements. ‘f’ block
elements in 2 rows [4f lanthanides 5f
actinides]
(C) Helium is placed ns2. But it has Noble
gas configuration.
General electronic configuration :
Screening effect is also known as the
shielding effect. The phenomenon which
occurs when the nucleus reduces its force
of attraction on the valence electrons due
to the presence of electrons in the inner-
shell. This is known as a screening
effect.
Effective nuclear charge (Z*) = Nuclear
charge Shielding effect
Trend Left to Right Z* Increases. Top to
Bottom Z* Decreases.
Second period element Show different
behavior that I group element due to (a)
small size (b) High electron negativity (c)
High polarising power (d) absence of ‘d’
orbital in I member. Na3[Al(OH)6] exists
but Na[B(OH)4] not exists.
A diagonal relationship is said to exist
between certain pairs
of diagonally adjacent elements in the
second and third periods of the periodic
table. Diagonal relationships occur
because of the directions in the trends of
various properties as you move across or
down the periodic table.
Periodic Table and Periodicity in Properties
3](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-14-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
2 | P a g e CHEMISTRY (313)
• Elements with outer shell:
a. 1, 2, 3, e - metals
b. 4e - metalloids
c. 5, 6, 7, e - non-metals
d. 8e - noble gas
Atomic radius: It is one-half the
distance between the nuclei of two
atoms
Atomic radius decreases from left to
right within a period. This is caused by
the increase in the number of protons
and electrons across a period.
Atomic radius increases from top to
bottom within a group. This is caused by
electron shielding.
Noble gases large radius than group 17
due to complete filling of electron in
outer shell electron-electron repulsion
mildly increases.
Ionization energy is the energy
required to remove an electron from a
neutral atom in its gaseous phase.
The ionization energy of the elements
within a period generally increases from
left to right. This is due to valence shell
stability.
The ionization energy of the elements
within a group generally decreases from
top to bottom. This is due to electron
shielding.
The noble gases possess very high
ionization energies because of their full
valence shells as indicated in the graph.
Note that helium has the highest
ionization energy of all the elements.
Metallic behavior: Decrease from left to
right due to increase in ionization
enthalpy.
Non metallic behavior: Increase from left
to right due to more number of electrons in
outershell and added electron goes
towards nucleus.
Ionic radius: Cation radius < Atomic
radius due to more no. of protons than
number of electron columbic force
increases size decreases. [Mg2+ < Mg+1 <
Mg]
Anion radius > Atomic radius due to more
number of electron than number of
protons. [N3- > O2- > F-] Electron-Electron
repulsion increases, coloumbic force of
attraction decreases.
For Isoelectronic species more is the
charge of cation lesser in the size.
Electronegativity: It can be understood as
a chemical property describing an atom's
ability to attract and bind with electrons.
From left to right across a period of
elements, electro negativity increases.
From top to bottom down a group, electro
negativity decreases.
Important exceptions of the above rules
include the noble gases, lanthanides, and
actinides.
Electron Affinity: As the name suggests,
electron affinity is the ability of an atom to
accept an electron.
Electron affinity increases from left to right
within a period. This is caused by the
decrease in atomic radius.
Electron affinity decreases from top to
bottom within a group. This is caused by
the increase in atomic radius.](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-15-320.jpg)



![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
1 | P a g e CHEMISTRY (313)
The interaction between two atoms which
holds them together within a molecule or
ions in known as chemical bond.
The elements with one, two, three, four,
five, six or seven electrons is outer shell,
use these electrons to complete octet.
The electrons which take part in two or
more atoms to complete octet is known as
electrovalency.
Lewis symbols or electron dot symbols
involve the presentation of valence
electrons (outer electrons) in an atom
Electrovalent bond or ionic Bond: The
bond (chemical interaction) between two
atoms formed by complete transference of
electron from valence shell (outer shell) of
an atom to another to complete octet
(noble gas configuration) [2e- in H, Li] is
known as ionic bond.
This ionic bond is favored by low
ionization enthalpy of metal high electron
gain enthalpy of non-metal atom and in
the resulting ionic compound more lattice
energy.
Characteristics of ionic compound:
They are solids, a definite arrangement/
pattern of ion (to give crystalline solids),
high MP and BP, conductors in fused
state and in aqueous medium, soluble in
H2O [Hydration].
Octate rule: During a chemical reaction
the atoms tend to adjust their electronic
arrangement in such a way that they
achieve 8 e- in their outermost electron.
Chemical bond: the chemical force which
keeps the atoms in any molecule together
is called a chemical bond.
Ionic bond: An ionic bond is formed by
the complete transfer of one or more
electrons from the atom of a metal to an
atom of non- metal.
Lattice enthalpy: It is a measure of the
strength of the forces between the ions in an
ionic solid. The greater the lattice enthalpy,
the stronger is the forces.
Electro valency: The number of electrons
lost or gain by an atom of an element is
called as electrovalency.
Covalency: The number of electrons which
an atom contributes towards mutual sharing
during the formation of a chemical bond.
Single covalent bond: A covalent bond
formed by the mutual sharing of one pair of
electrons is called a single covalent bond. It
is represented by a small line (−) between
the two atoms.
Double covalent bond: A covalent bond
formed by the mutual sharing of two pair of
electrons is called a double covalent bond. It
is represented by two small horizontal lines
(=) between the two atoms. E.g. O=O,
O=C=O etc.
Triple covalent bond: A covalent bond
formed by the mutual sharing of three pair of
electrons is called a triple covalent bond. It is
represented by three small horizontal lines
(≡) between the two atoms. E.g. N≡N, H-
C≡C-H etc.
Polar covalent bond: The bond between
two unlike atoms which differ in their affinities
for electrons is said to be polar covalent
bond. E.g. H-Cl
Coordinate bond: The bond formed
sided sharing of electrons take place is
called a coordinate bond. It is represented by
an arrow (→) pointing towards the acceptor
Chemical Bonding
4](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-20-320.jpg)

![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
3 | P a g e CHEMISTRY (313)
(b) The strength of covalent bond is
proportional to extent of overlapping
between the atomic orbitals of valence
shell.
• Sigma and Pi bond: (a) The bond
formed by overlap of two atomic
orbitals along the internuclear axis of
two atoms is Sigma bonds.
Summary of the chapter
Fig.4.1: Formation of Sigma and Pi bond
• Dipole moment: (a) For polar covalent
molecules (atoms with difference in
electronegativity] the product of charge
separation and distance b/w charges is
known as dipole moment.
(b) Being vector, if net resultant of all
vectors is zero the molecule has zero
DM and known as non polar otherwise.
• Hydrogen bonding: The dipole
interaction b/w molecules when H is
bounded wih highly electronegtive
atoms (F, O, N only).
(a) Intermolecular HB: When hydrogen
bonding is in between the same
molecule. Hence molecules are
independent and with less MP, BP. Due
to within hydrogen bonding notable to
make hydrogen bonding with H2O therefore
less soluble in water.
(b) Intermolecular hydrogen bonding: When
hydrogen bonding in between the different
molecules therefore close packing of
molecules therefore more MP and BP and
more soluble in water.
• Molecular orbital theory : (a) The overlap
of atomic orbitals of same symmetry of two
homonuclear atoms to give addition or
subtraction of wave functions and form
bonding MO and antibonding MO
respectively is known as MO theory.
(b) The e- is filled in molecule increasing energy
order of MO.
(c) Bond order: Bond order of molecule/ion.
(d) More is bond order more is bond energy
lesser is the bond length. Bond order zero
means no. possibility of that molecule. (e)
Increasing order of energy of MO for upto
14e-.
(e) This theory decides the magnetic behaviour
also. [Equal energy orbitals]
• Coordinate covalent bond: The sigma
bond formed by donation of lp into vacant by
drized orbital of other atom (acception atom)
is known as coordinte covalent bond or
donor acceptor or daive bond.](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-22-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
4 | P a g e CHEMISTRY (313)
• Hybridisation (or hybridization) is a
proces of mathematically combining two
or more atomic orbitals from the same
atom to form an entirely new orbital
different from its components and hence
being called as a hybrid orbital.
• Valence shell electron pair
repulsion theory, or VSEPR theory),
is a model used in chemistry to predict
the geometry of individual molecules
from the of electron pairs surrounding
their central atoms.
• Molecular orbital theory (MO
theory or MOT): It is a method for
describing the electronic structure of
molecules using quantum mechanics. It
was proposed early in the 20th century.
Molecular orbital theory and valence
bond theory are the
foundational theories of quantum
chemistry.
• The Born-Haber cycle: It is a classic
approach to measure the reaction
energies. The cycle is mostly concerned
with the formation of an ionic solid from
the metals (Group I or Group II) when
reacted with a halogen or a non-metallic
element like oxygen.
• Valence bond theory: It is
a theory which describes chemical
bonding. VBT states that the overlap of
incompletely filled atomic orbitals
leads to the formation of a chemical bond
between two atoms. The unpaired
electrons are shared and a hybrid orbital
is forsmed.
• Bond order = [(Bonding molecules'
number of electrons) – (Antibonding
molecules' number of electrons)]/2.
I.e. Bond Order = ½ [Nb – Na] Where, Nb
is that the number of bonding electrons.
• Lewis Structure: Itis a very simplified
representation of the valence shell
electrons in a molecule. It is used to show
how the electrons are arranged around
individual atoms in a molecule. Electrons
are shown as "dots" or for bonding
electrons as a line between the two atoms](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-23-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
5 | P a g e CHEMISTRY (313)
Q. Draw all the atomic models given
in your textbook and compare which
one is the correct explanation for the
atomic structure.
Also discuss the Drawbacks of
some atomic models.
Test Yourself
1. C-O bond length is minimum in
(A) CO2 (B) CO3
2-
(C) HCOO– (D) CO
2. Molecules are held together in a
crystal by
(A) Hydrogen bond
(B) Electrostatic attraction
(C) Van der Waal’s attraction
(D) Dipole-dipole attraction
3. Sp3d2 hybridization is present in
[Co (NH3)6
3+], find its geometry
(A) Octahedral geometry
(B) Square planar geometry
(C) Tetragonal geometry
(D) Tetrahedral geometry
4. Find the molecule with the
maximum dipole moment
(A) CH4 (B) NH3 (C) CO2 (D) NF3
5. MX6 is a molecule with octahedral
geometry. How many X – M – X bonds
are at 180°?
(A) Four (B) Two
(C) Three (D) Six
Check Yourself
1. Explain the non linear shape of H2S
and non planar shape of PCl3 using
valence shell electron pair repulsion
theory.
2. Using molecular orbital theory,
compare the bond energy and
magnetic character of O2+ and O2–
species.
3. Explain the shape of BrF5.
4. Explain why PCl5 is trigonal
bipyramidal whereas IF5 is square
pyramidal.
5. In both water and dimethyl ether
(CH3— Ö — CH3), oxygen atom is
central atom, and has the same
hybridization, yet they have different
bond angles.
Which one has greater bond angle?
Give reason.
Stretch Yourself](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-24-320.jpg)



![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
3 | P a g e CHEMISTRY (313)
Question: A balloon is filled with
hydrogen at room temperature. It
will burst if pressure exceeds 0.3
bar. If at 1 bar pressure the gas
occupies 3.27 L volume, upto what
volume can the balloon be
expanded?
Answer: According to Boyle’s Law
p1V1 = p2V2 If p1 is 1 bar, V1 will be
3.27 L If p2 = 0.3 bar, then V2=
p1V1/p2 or V2= 1bar×3.27L/0.3bar ⇒
= V2 =10.9 L Since balloon bursts at
0.2 bar pressure, the volume of
balloon should be less than 10.9 L.
Test Yourself
1. What are the conditions for gas like
Carbon monoxide to obey the ideal
gas laws?
(A) Low temperature and low pressure
(B) Low temperature and high
pressure
(C) High temperature and low
pressure
(D) High temperature and high
pressure
2. If the temperature is doubled, the
average velocity of a gaseous
molecule increases by
(A) 4 (B) 1.4 (C) 2 (D) 2.8
3. At the same temperature, the
average molar kinetic energy of N2
and CO is
(A) KE1 > KE2 (B) KE1 < KE2
(C) KE1 = KE2
(D) Insufficient information given
4. Find the temperature at which the
rate of effusion of N2 is 1.625 times to
that of SO2 at 500℃
(A) 620℃ (B) 173℃
(C) 110℃ (D) 373℃
5. Find the fraction of the total
pressure exerted by hydrogen if it is
mixed with ethane in an empty
container at 25℃
(A) 15/16 (B) 1/16 (C) 1/2 (D) 1
Check Yourself
1. Define the terms:
(i) Standard boiling point.
(ii) Vapor pressure of a liquid.
2. Drops of liquid are spherical in
nature. Explain. Mention the effect of
temperature on surface tension.
3. Write the S.I. units of:
(i) Surface tension.
(ii) Coefficient of viscosity.
4. 300 ml of oxygen gas at ñ 10°C are
heated to 10°C. Find the volume of
gas at 10°C if pressure remains
constant. [Ans. 322.8 mL]
5. A gas at a pressure of 5 atm is
heated from 0° to 546°C nd is
simultaneously compressed to one
third of its originl volume. Find the final
pressure f the gas.
Stretch Yourself](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-30-320.jpg)


![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
1 | P a g e CHEMISTRY (313)
Solids: Solids are the chemical substances
which are characterized by define shape and
volume, rigidity, high density, low
compressibility. The constituent particles
(atoms, molecules or ions) are closely packed
and held together by strong interparticle forces.
Types of Solids: The solids are of two types:
Crystalline solids and amorphous solids.
Crystalline solids: consist of atoms, ions and
molecules arranged in definite and repeating
three-dimensional patterns in a highly ordered
microscopic structure, forming a crystal lattice
that extends in all directions. Ex. salt (sodium
chloride), diamond, and sodium nitrate.
Amorphous solid, any noncrystalline solid in
which the atoms and molecules are not
organized in a definite lattice pattern.
Such solids include glass, plastic, and gel.
Structure Determination by X-ray Diffraction
(Bragg’s Equation): The law states that when
the x-ray is incident onto a crystal surface,
its angle of incidence, θ, will reflect back with a
same angle of scattering, θ. And, when the
path difference, d is equal to a whole number,
n, of wavelength, a constructive interference
will occur.
Unit Cell: The smallest geometrical portion of
the crystal lattice which can be used as
repetitive unit to build up the whole crystal is
called unit cell.
Types of unit cell:
Seven Crystal Systems: There are about 230
crystal forms, which have been grouped into 14
types of space lattices, called Bravais Lattices,
on the basis of their symmetry and seven
different crystal systems on the basis of
interfacial angles and axes.
They are cubic, tetragonal, hexagonal (trigonal),
orthorhombic, monoclinic, and triclinic.
Packing Fraction: It is defined as the ratio of
the volume of the unit cell that is occupied by
the spheres to the volume of the unit cell.
Packing fraction (P.F), is calculated by
volume occupied by the number of spheres in
the unit cell divided by volume of a unit cell.
Coordination Number: It is defined as the
number of particles immediately adjacent to
each particle in the crystal lattice. [In simple
cubic lattice, CN is 6, in body centered lattice,
CN is 8 and in face centered cubic lattice, CN is
12]. High pressure increases CN and high
temperature decreases the CN.
Close Packing in Crystals: Two Dimensional
Packing of Constituent Particles.
(i) Square close packing Space occupied by
spheres is 52.4%.
(ii) Hexagonal close packing Space occupied
by spheres is 60.4%.Hence. It is more efficient.
Three Dimensional Packing of Constituent
Particles:
(i) ABAB arrangement gives hexagonal close
packing (hcp).
(ii) ABCABC arrangement gives cubic close
packing or face centred CUbIC packing (ccp
or fcc).
In both these arrangements 740/0 space is
occupied
Coordination number in hop and ccp
arrangement is 12 while in bcc arrangement, it
is 8.
Close packing of atoms in cubic structure = fcc>
bcc > sc. All noble gases have ccp structure
except He (hcp structure).
The Gaseous State and Liquid State
6](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-33-320.jpg)








































![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
4 | P a g e CHEMISTRY (313)
Question: The concentration
of hydrogen ions in a sample
of soft drink is 3.8 x 10-3 M.
What is the pH value?
Answer: The pH of a solution
is the negative logarithm of
hydrogen ion concentration.
pH=−log[H3O+]
pH=−log(3.8×10−3)
pH=2.42
Test Yourself
1. Explain why pure liquids and
solids can be ignored while writing
the value of equilibrium constants.
2. The value of Kc for the reaction
302(g) —>203(g) is 2.0 x 10-50 at
25°C. If equilibrium concentration of
O2 in air at 25°C is 1.6x10-2, what is
the concentration of O3?
3. What is meant by conjugate acid-
base pair? Find the conjugate
acid/base for the following species:
HNO2, CH–, HClO4, OH–, CO3
2-, S2-
4. Which of the following are Lewis
Acids?
H2O, BF3, H+ and NH4+
5. What will be the conjugate bases
for the Bronsted acids? HF, H2SO4
and H2CO3?
Stretch Yourself
1. The solubility of Ca3(PO4)2 in water is
y mol/L. Its solubility product is:
(A) 6y² (B) 36 y4
(C) 64 y5 (D) 108 y5
2. Which of the following aqueous
solutions will have highest pH?
(A) NaCl (B) CH3COONa
(C) Na2CO3 (D) NH4Cl
3. What is the pH of a 0.10 M solution of
barium hydroxide, Ba(OH)2?
(A) 11.31 (B) 11.7
(C) 13.30 (D) None of these
4. Which of the following oxides is not
expected to react with sodium
hydroxide?
(A) CaO (B) SiO2
(C) BeO (D) B2O3
5. The pH of a 10-10 M NaOH solution is
nearest to
(A) 10 (B) 7
(C) 4 (D) -10
Check Yourself](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-76-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
5 | P a g e CHEMISTRY (313)
Answers
Check Yourself
Answer: 1(D); 2(C); 3(C); 4(A); 5(C)
Stretch Yourself
1. Pure solids and liquids are not included in the equilibrium constant expression.
This is because they do not affect the reactant amount at equilibrium in the
reaction, so they are disregarded and kept at 1.
2. 3O2 (g)=2O3 (g)
Equilibrium constant (Kc) =[O3]2/[O2]3
2×10−50= [O3]2/(1.6)3×10−6
[O3]2=2×10−50× (1.6)3×10−6=8.192×10−56
[O3]=√8.192×10−28
[O3]=2.86×10−28M
3. Conjugate acid base pairs differ by a proton.
For example, HCl, Cl− represents conjugate acid base pair.
The conjugate acid/base for the species HNO2, CN−,HClO4, F−,OH−,CO3
2− and S2−
are NO2−,HCN,ClO4−,HF,H2O (acid) or O2−(base), HCO3− and HS− respectively.
4. BF3, H+ and NH4
+ are Lewis acids whereas H2O is a lewis base.
5. The conjugate bases for the Bronsted acids HF, H2SO4 and HCO3
− are F−,
HSO4
− and CO3
2− respectively.](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-77-320.jpg)
![NIOS/Acad./2021/313/12/E
National Institute of Open Schooling
Senior Secondary Course: Chemistry
Chapter- 12 (Ionic Equilibrium)
Worksheet-12
1. BF3 does not have a proton but still acts as an acid and reacts with
NH3. Why it is so? What type of bond is formed between the two?
2. On the basis of the equation pH= - log [H+
], the pH of 10-8
mol dm-3
solution of HCl
should be 8. However, it is observed to be less than 7.0. Explain the reason.
3. The ionization constant of an acid, Ka, is the measure of the strength of an acid. The Ka
values of acetic acid, hypochlorous acid and formic acid are 1.74 × 10–5
, 3.0 × 10–8
and
1.8 × 10–4
respectively. Which of the following orders of pH of 0.1 mol dm–3
solutions of
these acids is correct?
a) acetic acid > hypochlorous acid > formic acid
b) hypochlorous acid > acetic acid > formic acid
c) formic acid > hypochlorous acid > acetic acid
d) formic acid > acetic acid > hypochlorous acid
4. A sparingly soluble salt having the general formula Ap+
xBq-
y and molar solubility S is in
equilibrium with its saturated solution. Derive a relationship between the solubility and
solubility product for such salt.
5. A crystal of common salt of a given mass is kept in an aqueous solution. After 12 hours,
its mass remains the same. Is the crystal in equilibrium with the solution?
6. From the values of the equilibrium constants, indicate in which case, does the reaction go
farthest to completion:
K1=10−10
, K2=1010
, K3=105
7. Following equilibrium is set up when SCN−
ion is added to Fe3+
in aqueous solution :
a) Fe3+
(Pale yellow) + SCN−
(Colourless) [Fe(SCN)]2+
( Deep red)
b) When silver nitrate is added to the solution, AgSCN gets precipitate. What will
happen to the equilibrium?
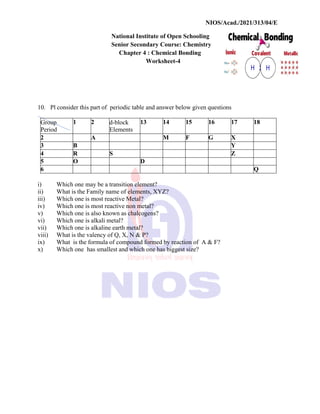
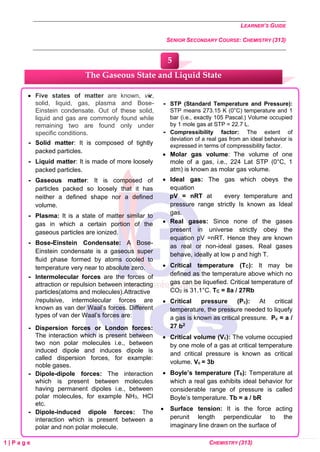
8. How will you account for the following:](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-78-320.jpg)




![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
4 | P a g e CHEMISTRY (313)
Question: Express the
relation between
conductivity and molar
conductivity of a solution
held in a cell.
Answer:
Test Yourself
1. Express the relation among cell
constant, resistance of the solution
in the cell and conductivity of the
solution. How is molar conductivity
of a solution related to its
conductivity?
2. The molar conductivity of a 1.5 M
solution of an electrolyte is found to
be 138.9 S cm2 mol-1. Calculate the
conductivity of this solution.
3. A zinc rod is dipped in 0.1 M
solution of ZnSO4. The salt is 95%
dissociated at this dilution at 298 K.
calculate the electrode potential.
[ E°Zn2+ /Zn = – 0.76 V]
4. Write the reactions taking place at
cathode and anode in lead storage
battery when the battery is in use.
What happens on charging the
battery?
5. The conductivity of 0.20 M
solution of KCl at 298 K is 0.025 S
cm-1. Calculate its molar
conductivity.
1. The charge required for the reduction of
1 mol of MnO4
–
to MnO2 is
(A) 1 F (B) 3 F (C) 5 F (D) 6 F
2. NH4NC3 is used in salt bridge because
(A) It forms a jelly like material with agar-
agar.
(B) It is a weak electrolyte.
(C) It is a good conductor of electricity.
(D) The transport number of NH4+
and
NO3–
ions is almost equal.
3. The reaction, 3ClO–
(aq) → ClO3 (aq) +
2Cl–
(aq) is an example of
(A) Oxidation reaction
(B) Reduction reaction
(C) Disproportionation reaction
(D) Decomposition reaction
4. The emf of the cell:
Ni / Ni2+
(1.0 M) // Au3+
(1.0 M) / Au (E° = -
0.25 V for Ni2+/
Ni; E° = 1.5 V for Au3+/
Au)
is
(A) 1.25 V (B) -1.25 V
(C) 1.75 V (D) 2.0 V
5. If E°Fe2+/
Fe = -0.441 V and E°Fe2+/
Fe2+
= 0.771 V, the standard EMF of the
reaction,
Fe + 2Fe3+
→ 3Fe2+
will be
(A) 1.212 V (B) 0.111 V
(C) 0.330 V (D) 1.653 V
Check Yourself
Check Yourself](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-83-320.jpg)



![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
2 | P a g e CHEMISTRY (313)
First Order Reaction
• A first-order reaction is a chemical
reaction in which the rate varies based
on the changes in the concentration of
only one of the reactants.
• Integrated rate law equation for first
order reaction :
Where, k is rate constant, [R]0 is
initial molar concentration and [R] is
final concentration at time 't'.
(b) Half-life period (t 1/2) for first order reaction:
• Pseudo chemical reaction: The
chemical reaction which looks like
higher order reaction but in real it
follows lower order reaction.
COLLISION THEORY OF
REACTION RATES
• According to this theory, the reactant
molecules are assumed to be hard
spheres and the reaction is postulated
to occur, when molecules collide with
each other.
• The number of collisions between the
reacting molecules taking place per
second per unit volume is known as
collision frequency (ZAB)·
• But only those collisions in which the
colliding species are associated with
certain minimum amount of energy and
collide in proper orientation result in the
product formation, such collisions are
called fruitful collisions or effective
collision.
• Here, rate = – (dv/dt) = collision
frequency x fraction of effective collision
= ZAB x f = ZAB x e-E a /RT
DEPENDENCE OF REACTION
RATE ON TEMPERATURE
Arrhenius Equation: Arrhenius
equation is a mathematical expression
to give a quantitative relationship
between rate constant and temperature,
and the expression is:
Fig. 14.3 Graphical determination of Ea.
Where, A = frequency or Arrhenius factor.
It is also called pre-exponential factor, R
= gas constant and Ea = activation
energy
• Half-life period: The time during which
the concentration of the reactant is
reduced to half of its initial concentration
is called half-life period.
• Activated complex (or transition
state): Activated complex is the highest
energy unstable intermediate between
the reactants and products and gets
decomposed immediately (having very
short life), to give the products. In this
state, bonds of reactant are not
completely broken while the bonds of
products are not completely formed.
Fig. 14.2 Energy diagram for a reaction](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-87-320.jpg)

![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
4 | P a g e CHEMISTRY (313)
Question: What do you
understand by the rate law and
rate constant of a reaction?
Identify the order of a reaction if
the units of its rate constant are:
(i) L-1 mol s-1 (ii) L mol-1 s-1
Answer: Rate=k [A]x [B]y
⇒ Order=x+y
The reaction order from each of
the following units of reaction rate
constant:
(i)L−1mols−1: Zero order
(ii)Lmol−1s−1: Second order
Test Yourself
1. If the rate constant of a reaction is
k = 3 × 10-4 s-1, then identify the
order of the reaction.
2. For a reaction R → P, half-life
(t1/2) is observed to be independent
of the initial concentration of
reactants. What is the order of
reaction?
3. A reaction is of second order with
respect to a reactant. How will the
rate of reaction be affected if the
concentration of this reactant is
(i) Doubled, (ii) Reduced to half?
4. The rate constant for a reaction of
zero order in A is 0.0030 mol L-1 s-1.
How long will it take for the initial
concentration of A to fall from 0.10
M to 0.075 M?
5. Distinguish between ‘rate
expression’ and ‘rate constant’ of a
reaction.
Stretch Yourself
1. What will be the fraction of
molecules having energy equal to or
greater than activation energy, Ea?
(A) K (B) A
(C) Ae-Ea/Rt (D) e-Ea/Rt
2. For a chemical reaction A→B, it is
found that the rate of reaction doubles
when the concentration of A is
increased four times. The order of
reaction is
(A) Two (B) One
(C) Half (D) Zero
3. The half life of the first order
reaction having rate constant K = 1.7 x
10-5s-1 is
(A) 12.1 h (B) 9.7 h
(C) 11.3 h (D) 1.8 h
4. The rate of a chemical reaction tells
us about
(A) The reactants taking part in the
reaction
(B) the products formed in the reaction
(C) How slow or fast the reaction is
taking place
(D) None of the above
5. The average rate and instantaneous
rate of a reaction are equal
(A) At the start (B) At the end
(C) In the middle
(D) When two rates have a time
interval equal to zero
Check Yourself](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-89-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
5 | P a g e CHEMISTRY (313)
Answers
Check Yourself
Answer: 1(D); 2(C); 3(C); 4(C); 5(B)
Stretch Yourself
1. On the basis of unit of rate constant (s-1), the order of the reaction is first order.
2. Second Order
3. 1. A reaction is second order with respect to a reactant.
Rate =k[A]2
(i) If the concentration of the reactant is doubled, the rate of reaction becomes 4 times.
(ii) If the concentration of the reactant is reduced to half, the rate of reaction becomes one
fourth.
4. For a zero order reaction
5. Do it by yourself.](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-90-320.jpg)

















![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
3 | P a g e CHEMISTRY (313)
Sodium Carbonate
• Manufacturing of washing soda
(Na2CO3.10H2O) :
• Solvay process :
Sodium hydrogen carbonate
• This can be obtained by passing carbon
dioxide through a cold concentrated
solution of the corresponding carbonate,
e.g.
Calcium oxide (CaO)
• Manufacture of CaO: CaO (quick lime) is
manufactured in enormous quantities (126
million tonnes in 1988) by roasting CaCO3
in lime Kiln.
CaCO3 Calcium Carbonate
• CaCO3 occurs in two different crystalline
forms, calcite and aragonite. Both forms
occur naturally as minerals. Calcite is the
more stable: each Ca2+ is surrounded by
six oxygen atoms from CO32– ions.
Uses of Lime:
1. In steel making to remove phosphates and
silicates as slag.
2. By mixing with SiO2 and alumina or clay to
make cement.
Diagonal relationship (similarities)
between Be and Al:
i. Both are passive to acids due to formation
of oxide layer.
ii. Hydroxides of both dissolve in alkali to
form [Be(OH)4]2- and [Al(OH)4]-.
iii. Chloride of both has bridged structure.
iv. Both have tendency of form complexes
of BeF4
2-, AlF6
3-.
• Solution in liquid ammonia: The fresh
solution of alkali metals and alkaline
earth metals (except Be and Mg) is deep
blue, paramagnetic and highly reducing
due to presence of ammoniated
electrons.
• Solubility of alkaline earth metal
hydroxide in water :
Li2CO3 < Na2CO3 < K2CO3<RbCO3 < Cs2 CO3
• Solubility of alkaline earth metl
carbonates in water.
BaCO3 < SrCO3 < CaCO3 < MgCO3 < BeCO3
• Solubility of alkaline earth metal
sulphates in water :
BaSO4 < SrSO4 < CaSO4 < MgSO4 < BeSO4
• Thermal stability of alkali metal
carbonates:
Li2CO3 < Na2CO3 < K2CO3 < Rb2CO3 < Cs2CO3
• Thermal stability of alkaline earth metal
carbonates :
BeCO3 < MgCO3 < CaCO3 < SrCO3 < BaCO3
• Biological Role of Mg2+ and Ca2+
• Mg2+ ions are concentrated in animal
cells, and Ca2+ are concentrated in the
body fluids outside the cell.
• They are also essential for the
transmission of impulses along nerve
fibres. Mg2+ is important in chlorophyll,
in the green parts of plants.
• Ca2+ is important in bones and teeth as
apatite Ca3(PO4)2, and the enamel on
teeth as fluoroapatite [3(Ca3(PO4)2)•
CaF2].
• Ca2+ ions are important in blood clotting,
and to maintain the regular beating of
the heart.](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-110-320.jpg)















![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
6 | P a g e CHEMISTRY (313)
(i) It is a colourless and odourless gas.
(ii) It is slightly soluble in water. When
C02 dissolves in water only some of the
molecules react with water to form
carbonic acid.
(iii) It is not poisonous like CO.
But increase in combustion of fossil fuels
and decomposition of limestone for
cement manufacture increase of C02 in
the atomosphere is one of the main
reasons of green house effect.
Silicon dioxide (Si02) Silicon dioxide,
commonly known as silica, occurs in
various crystallographic forms. For
example, Quartz, Cristobalite and
thermite are some of the crystalline
forms of silica.
Structure: Silicon dioxide is a covalent
three dimensional network solid.
Each silicon atom is covalently bonded in
a tetrahedral manner to four oxygen
atoms. Each oxygen atom in turn
covalently bonded to another silicon
atoms as shown below:
Properties:
(i) In normal form silica is very less reactive.
(ii) At elevated temperature it does not
reacts with halogens, dihydrogen and
most of the acids and metals. But it
reacts with HF and NaOH.
SiO2 + 2NaOH —–> Na2SiO3 + H2O
SiO2+ 4HF ——–> SiF4+ 2H2O
Uses:
(i) Quartz is extensively used as a
piezoelectric material.
(ii) Silica gel is used as adsorbent in
chromatography.
(iii) An amorphous form of silica, kieselghur is
used in filtration plants.
Group 15 Elements
Physical Properties
Electronic configuration of group 15
members: general electronic configuration
is ns2np3.
o Nitrogen (N) [He]2s2,2p3
o Phosphorous (P) [Ne]3s2,3p3
o Arsenic(As) [Ar]3d10,4s2,4p3
o Antimony (Sb) [Kr]4d10,5s2,5p3
o Bismuth (Bi) [Xe]4f14,5d10,6s2,6p3
Atomic size: As compared to group 14
they are smaller in size due to increased
nuclear charge. Along group, size increases
as every time a new shell is being added
due to which nuclear charge decreases.
Ionization energy: The group 15 has high
ionization energy than group 14 because of
smaller size. Along group, ionization energy
decreases as size increases
Electro negativity: Because of smaller size
the group 15 members re more
electronegative than group 14. Out of them
the increasing order of electro negativity is:
N>P>As>Sb>Bi
Metallic character: They are less metallic
than group 14 because of small size and
increased nuclear charge. Along group
metallic character increases as size
increases and ionization energy decreases.
The order of their metallic character is:
N<P<As<Sb<Bi
Melting point and boiling point: The
melting point depends upon the type and
number of bonds formed whereas boiling
point depends upon Vander wall force
which increases in magnitude with increase
in size.
o Boiling points: It increases down the group
as size increases. The order is –
N<P<As<Sb<Bi
o Melting point: It first increases then
decreases. The order is: N<P<As>Sb>Bi](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-128-320.jpg)













![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
2 | P a g e CHEMISTRY (313)
In contrast to s-and p-block elements,
the transition elements have the ability
to form complexes. This is because
these elements
(a) Have small highly charged ions, and
(b) Contain vacant d-orbitals.
Many of transition metals and their
compounds act as catalyst in variety of
reactions.
Transition metals form large number of
interstitial compounds.
A large number of alloys are formed by
transition metals. It is due to their
atoms mutually substitute their
positions easily in their metal crystal
lattices.
The oxides of transition metals in lower
oxidation states are generally basic in
nature and those in higher oxidation
states are amphoteric or acidic in
nature.
f-Block Elements
The f -block elements have been
divided in two series depending upon
the fact whether the last electron
(differentiating electron) enters 4f-
orbitals or 5f-orbitals and accordingly
called lanthanides or actinides
respectively.
Actinides show several oxidation states
but + 3 oxidation state is most
common. The highest oxidation state
shown by actinides is + 7.
Properties of the lanthanides:
(a) General electronic configuration is [Xe]
4f1-14 5d0-1 6s2.
(b) The metals are silvery-white in colour.
They are malleable, ductile, have low
tensile strength and are good
conductors of heat and electricity.
(c) They have relatively high density and
possess high melting points.
(d) The lanthanides exhibit a principle oxidation
state of +3. However, some elements also
exhibit +2 (Eu2+) and +4 (Ce4+) oxidation
states.
(e) Many of the lanthanide ions are coloured
due to the electronic transition between
different 4f levels.
(f) The majority of the lanthanide ions exhibit
paramagnetism due to the presence of
unpaired electrons. The lanthanoid ions
that do not exhibit paramagnetism are
those with either no 4f-electrons, e. g.,
La3+ and Ce4+ or with a completed 4f-level,
e.g., Yb2+ and Lu3+.
(g) The lanthanides readily tarnish in air and
bum to give trioxides (except cesium, which
forms Ce02).
(h) The oxides and hydroxides of the
lanthanides are basic in character.
(i) The lanthanoid compounds are generally
predominantly ionic.
This gradual decrease in atomic size
across the first f- transition element series
is called lanthanoid contraction.
Properties of actinides:
General electronic configuration is [Rn] 5f0-
14 6ds0-1 7s2.](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-144-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
3 | P a g e CHEMISTRY (313)
The elements are all silvery-white
metals.
The melting points of the actinides are
moderately high.
The ionic size of the actinides
decreases gradually along the series.
The actinides have the ability to exhibit
several oxidation states. However, +4
oxidation state is preferred in actinides.
Some actinoid elements can exist in +
6 oxidation state, e.g., uranium,
neptunium and plutonium.
Many actinoid elements are
radioactive. The elements beyond
uranium are man-made.
The actinides have a much greater
tendency to form complexes than
lanthanides.
Question: Write down the
electronic configuration of Cr3+
Answer: Chromium has atomic
number 24. So, nearest noble gas
element is Argon (Ar) So electronic
configuration of Cr3+ = [Ar]183d34s0
Test Yourself
1. Which of the following has magnetic
moment value of 5.9?
(A) Fe2+ (B) Fe3+
(C) Ni2+ (D) Cu2+
2. Anomalous electronic configuration in
the 3d series are of
(A) Cr and Fe (B) Cu and Zn
(C) Fe and Cu (D) Cr and Cu
3. Which of the following are d-block
elements but not regarded as
transistion elements?
(A) Cu, Ag, Au (B) Zn, Cd, Hg
(C) Fe, Co, Ni (D) Ru, Rh, Pd
4. Which of the following has the
maximum number of unpaired
electrons?
(A) Mg2+ (B) Ti3+
(C) V3+ (D) Fe2+
5. The property which is not
characteristic of transistion metals is
(A) Variable oxidation states.
(B) Tendency to form complexes.
(C) Formation of coloured compounds.
(D) Natural radioactivity.
Check Yourself](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-145-320.jpg)




![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
2 | P a g e CHEMISTRY (313)
defined as the number of ligand
(donor) atoms/ions surrounding the
central metal atom in a complex ion.
For example, the coordination number
of cobalt in [Co(NH3)6]3+ is six.
Coordination sphere: The central
metal atom and the ligands which are
directly attached to it are enclosed in
a square bracket and are collectively
termed as coordination sphere.
o
Oxidation number: The net charge
on a complex ion is the sum of the
charges on the central atom and its
surrounding ligands. In the [PtCl6]2-
ion for example, each chloride ion has
an oxidation number of –1, so the
oxidation number of Pt must be +4.
Paramagnetism due to presence of
unpaired electrons, each such
electron having a magnetic moment
associated with its spin angular
momentum.
RULES OF NOMENCLATURE OF
COORDINATION COMPOUNDS
Cationic Complex:
i. Prefixes mono, di, tri, etc. are used to
indicate the number of the individual
ligands and ligands are named in an
alphabetical order.
ii. Central metal atom and oxidation
state indicated by Roman numeral in
parenthesis.
iii. Name of ionisable anion
Anionic Complex:
(i) Name of ionisable metal and oxidation
state.
(ii) Name of ligand in an alphabetical order
(iii) Central metal atom + ate and oxidation
state
1. Name of ligands in an alphabetical order
2. Central metal atom and oxidation state
VALENCE BOND THEORY
According to this theory, the metal atom
or ion under the influence of ligands form
inner orbital and outer orbital complex.
These hybridized orbitals are allowed to
overlap with ligand orbitals that can
donate electron pairs for bonding.
(i) Six ligands (unidentate) (octahedral entity)
Generally central atom belongs 3d series
and ligands can be monodentate or
didentate but coordination number
should be six and shape of complexes
will be octahedral and form two types of
complexes.
(a) Inner orbital complexes, which are
formed due to participation of (n-1)d
orbitals in hybridisation is (d2sp3) and
shape of complex will be octahedral.
(b) Outer orbital complexes, which are
formed due to participation of nd orbitals
in hybridisation is (sp3d2). Generally
halides (F −, Cl −, Br −, I −), SCN −, S2−
form outer orbital complexes and other
ligands form inner orbital complexes.](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-150-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
3 | P a g e CHEMISTRY (313)
All electrons are paired therefore,
complex will be diamagnetic in nature.
Complex has unpaired electrons;
therefore, complex will be
paramagnetic in nature.
Generally halides (F−, Cl−, Br−, I− )
ligands, [Ni(CO)4], [Co(CO)4],
[Zn(NH3)4]2+ complexes form outer
orbital complexes and other form inner
orbital complexes. For example,
1. Inner orbital complex, [Ni(CN)4]−2
2. Outer orbital complex, [CoCl4]−
CRYSTAL FIELD THEORY
The five d-orbitals are split into lower and
higher energy level due to approach of
ligands is known as crystal field theory.
The five d-orbitals in a gaseous metal
atom/ion have same energy.
(i) Crystal field splitting in octahedral
coordination entities.
Energy separation is denoted by o (the
subscript o is for octahedral).
The energy of the two eg orbitals (higher
energy orbitals) will increase by (3/5)o
and that of the three t2g (lower energy
orbitals) will decrease by (2/5)o.
If o<p, the fourth electron enters one of
the eg, orbitals giving the configuration
Ligands for which o<p are known
as weak field ligands and form high spin
complexes.
If o>p, it becomes more energetically
favorable for the fourth electron to
occupy a t2g orbital with configuration
Ligands which produce this effect](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-151-320.jpg)

![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
5 | P a g e CHEMISTRY (313)
Question: Using IUPAC norms
write the formulae for the following
coordination compounds:
(i) Tetracarbonylnickel(O)
(ii)Potassium tetracyanidoferrate(Il)
Answer:
(i) [Ni(CO)4]
(ii) K2[Fe(CN)4]
Test Yourself
1. Trunbull’s blue is
(A) Ferricyanide (B) Ferrous ferricyanide
(C) Ferrous cyanide (D) Fe3[Fe(CN)6]4
2. Primary and secondary valency of Pt in
[Pt(en)2Cl2] are
(A) 4, 4 (B) 4, 6
(C) 6, 4 (D) 2, 6
3. The complex ions [Co(NH3)5(NO2)]2+ and
[Co(NH3)5 (ONO)]2+ are called
(A) Ionization isomers
(B) Linkage isomers
(C) Co-ordination isomers
(D) Geometrical isomers
4. Which of the following has square planar
structure?
(A) [NiCl4]2- (B) [Ni(CO)4]
(C) [Ni(CN)4]2- (D) None of these
6. Which of the following has magnesium?
(A) Chlorophll
(B) Haemocyanin
(C) Carbonic anhydrate
(D) Vitamin B12
Check Yourself](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-153-320.jpg)
![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
6 | P a g e CHEMISTRY (313)
1. Why tetrahedral complexes
high are spin?
2. Write down the IUPAC
name of the complex
[Co(en)2Cl2]+. What type of
isomerism is shown by this
complex?
3. Predict the number of
unpaired electrons in
hexaaquamanganese(II)
ion. [Atomic number of Mn
= 25]
4. Write the chemical formula
and shape of hexa-
amminecobalt(III) sulphate.
5. A CuSO4 solution is mixed
with (NH4)2SO4 solution in
the ratio of 1:4 does not
give test for Cu2+ ion, why?
Stretch Yourself Answers
Check Yourself
Answer: 1(A); 2(C); 3(B); 4(D); 5(B)
Stretch Yourself
1. It is because of small splitting energy gap,
electrons are not forced to pair; therefore,
there is large number of unpaired electrons,
i.e. high spin.
2. Dichloro Bis-(ethane-l,2 diamine) Cobalt (III).
It will show geometrical as well as optical
isomerism.
3. It has 5 unpaired electrons.
4. [CO(NH3)6]2(SO4)3, octahedral.
5. [Cu(NH3)4]SO4 is formed which does not
have free Cu2+ ions.](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-154-320.jpg)

![NIOS/Acad./2021/313/22/E
National Institute of Open Schooling
Senior Secondary Course: Chemistry
Chapter 22: Coordination Compounds
Worksheet-22
6. Hard water does not form leathers with soap. Shalu uses a washing powder containing
sodium metapolyphosphate and ethylenediamine tetracetate (EDTA) while Komal is us-
ing ordinary washing power. Give your Suggestions.
(a) Which washing powder is move effective for washing clothes in hard water and why?
(b) Name the values associated with the above passage.
7. A lots of children, working in a lead industry were rescued by NGO’s activists. The chil-
dren were sent to the hospital and found to be excess exposure to lead so called lead poi-
soning. Give your Suggestions.
8. Write the IUPAC name for the following coordination compounds:
(i) [Co(NH3)4 (H2O)Cl]Cl2
(ii) [CrCl2(en)2]Cl,
(en = ethane – 1, 2 – diamine)
9. Describe the shape and magnetic behaviour of following complexes :
(i) [CO(NH3)6]3+
(ii) [Ni(CN)4]2-
(At. No. Co = 27, Ni = 28)
10. How is the stability of a co-ordination compound in solution decided?
How is the dissociation constant of a complex defined?](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-156-320.jpg)








![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
2 | P a g e CHEMISTRY (313)
(4) Cracking or Pyrolysis: At very high
temperature and in the absence of air,
the alkanes break apart into smaller
fragments.
(5) Isomerisation: n-Alkanes, in the
presence of aluminium halide and HCl, are
converted to their branched isomers.
Uses of Alkanes
Alkanes are important raw materials of
the chemical industry and the principal
constituent of gasoline and lubricating
oils. Natural gas mainly contains
methane and ethane and is used for
heating and cooking purposes and for
power utilities (gas turbines).
Alkenes
These are unsaturated hydrocarbons
containing at least one double bond
between two carbon atoms. The
hydrocarbons of this class are also
called olefines (olefiant = oil forming)
Methods of Preparation
(1) From alkynes [Alkyne + H2O →
Alkene]
(2) From alkyl halide by
(dehydrohalognation)
Carbon attached with halogen is -carbons
Carbon attached with -carbons is -
carbons
Halogen is removed and ‘H’-atom is
removed from - carbon to form (C = C)
double bond.
(3) By Dehydration of alcohols (Ion of water
molecule):
Carbon attached to alcohoic group is -
carbon.
Carbon attached to is -carbon - carbon
(4) From vicinal dihalides [Compounds in
which halogen atom are attached with
adjacent carbons]
Physical Properties of Alkenes
The boiling point of each alkene is very
similar to that of the alkane with the same
number of carbon atoms. Ethene, propene
and the various butenes are gases at room
temperature. All the rest that you are likely to
come across are liquids. Boiling points of
alkenes depends on more molecular mass
(chain length).
Chemical Properties of Alkenes
(1) Addition of Halogens:](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-166-320.jpg)

















































![LEARNER’S GUIDE
SENIOR SECONDARY COURSE: CHEMISTRY (313)
4 | P a g e CHEMISTRY (313)
(inert solvent) containing Ziegler-Natta
catalyst (a mixture of triethyl aluminium
and titanium chloride)
(3)Teflon or Polytetrafluoro ethylene
(PTFE): The monomer unit is
terafluoroethylene molecule. Teflon is
prepared by heating tetra fluoroethylene
under pressure in the presence of
ammonium peroxosulphate.
[(NH4)2S2O8].
(4) Polyvinylchloride (PVC): The
monomer units are vinyl chloride
molecules. PVC is prepared by heating
vinyl chloride in an inert solvent in the
presence of dibenzoyl peroxide.
(5) Polymethyl Methacrylate (PMMA): Its
monomer unit is methyl methacrylate.
Polyester
Some synthetic polymers have ester
group in them. These are
condensation polymers. The important
members of this class are polyester
and glyptal resins.
(a) Terelene: It is a polymer obtained by the
condensation reaction between ethylene
glycol and terephthalic acid.
(b) Glyptal or Alkyl resin: Glyptal is a general
name of all polymers obtained by
condensation of di-basic acids, and
polyhydroxy alcohols. The simplest glyptal is
(poly ethelene glycol phthalate) which is
obtained by a condensation reaction
between ethylene glycol and ortho-phthalic
acid.
Question: Name the important by-
products of soap industry.
Answer: Glycerol is the important
by-product of soap industry.
Test Yourself](https://image.slidesharecdn.com/chemistry-240808060318-da8c0e4a/85/NIOS-Class-12th-Chemistry-Notes-Class-12th-NIOS-222-320.jpg)