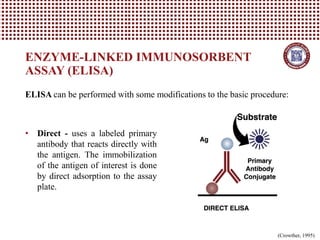
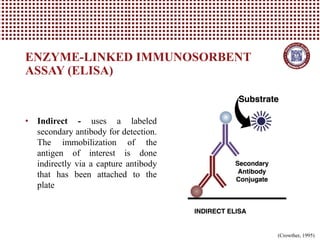
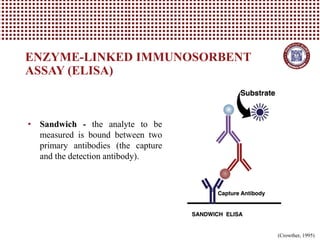
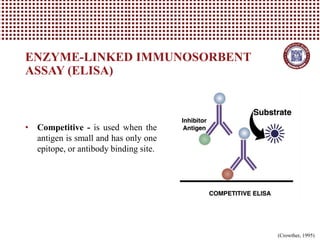
This document provides information on several common methods used to measure antioxidant capacity in biological samples and foods. It describes the principles, procedures, and key measurements of assays such as ORAC, DPPH, ABTS, Folin-Ciocalteu, FRAP, CAA, HPLC, UPLC, and ELISA. These assays utilize different chemical reactions and detection methods to quantify a sample's ability to inhibit oxidation initiated by free radicals.