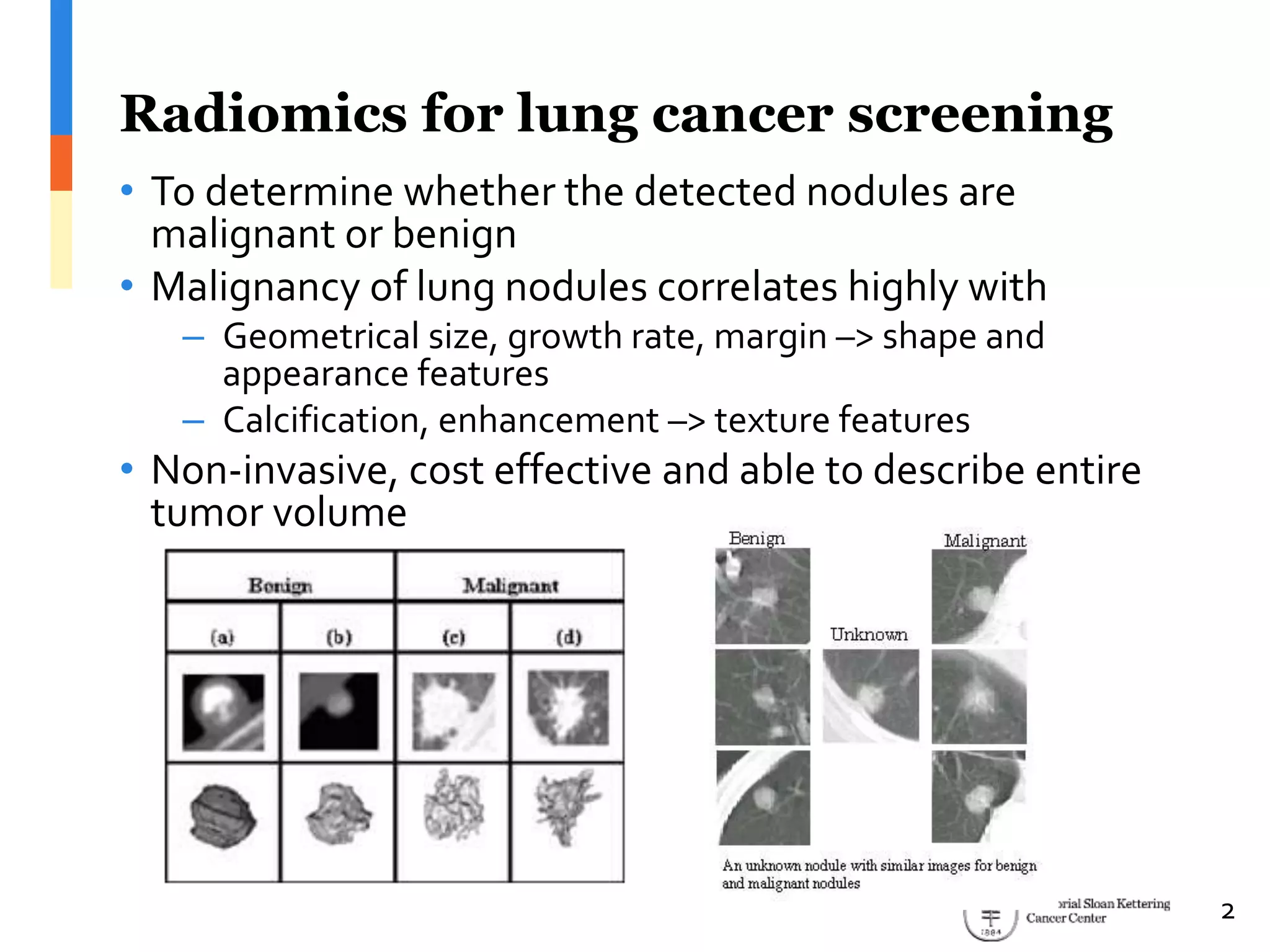
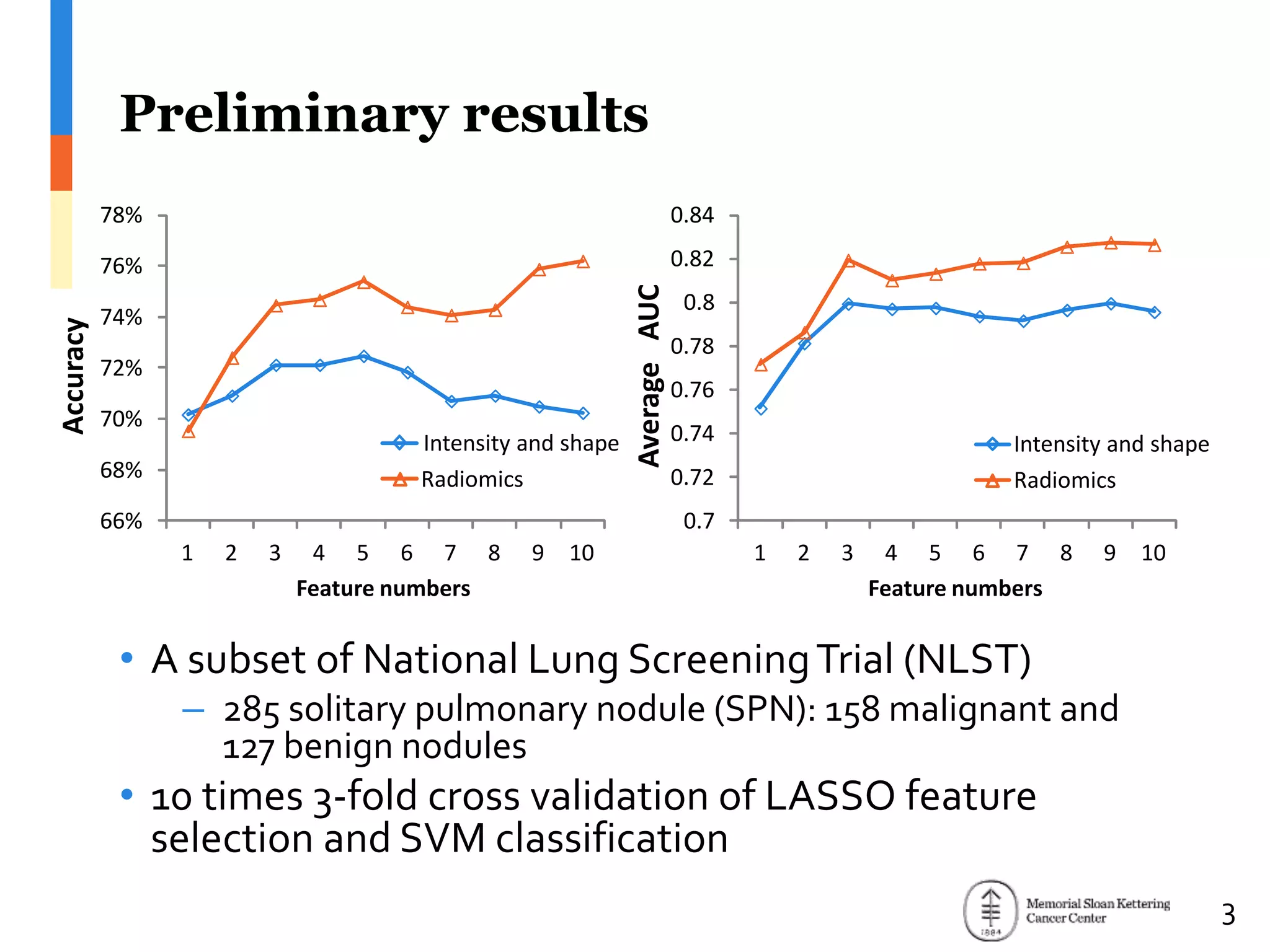
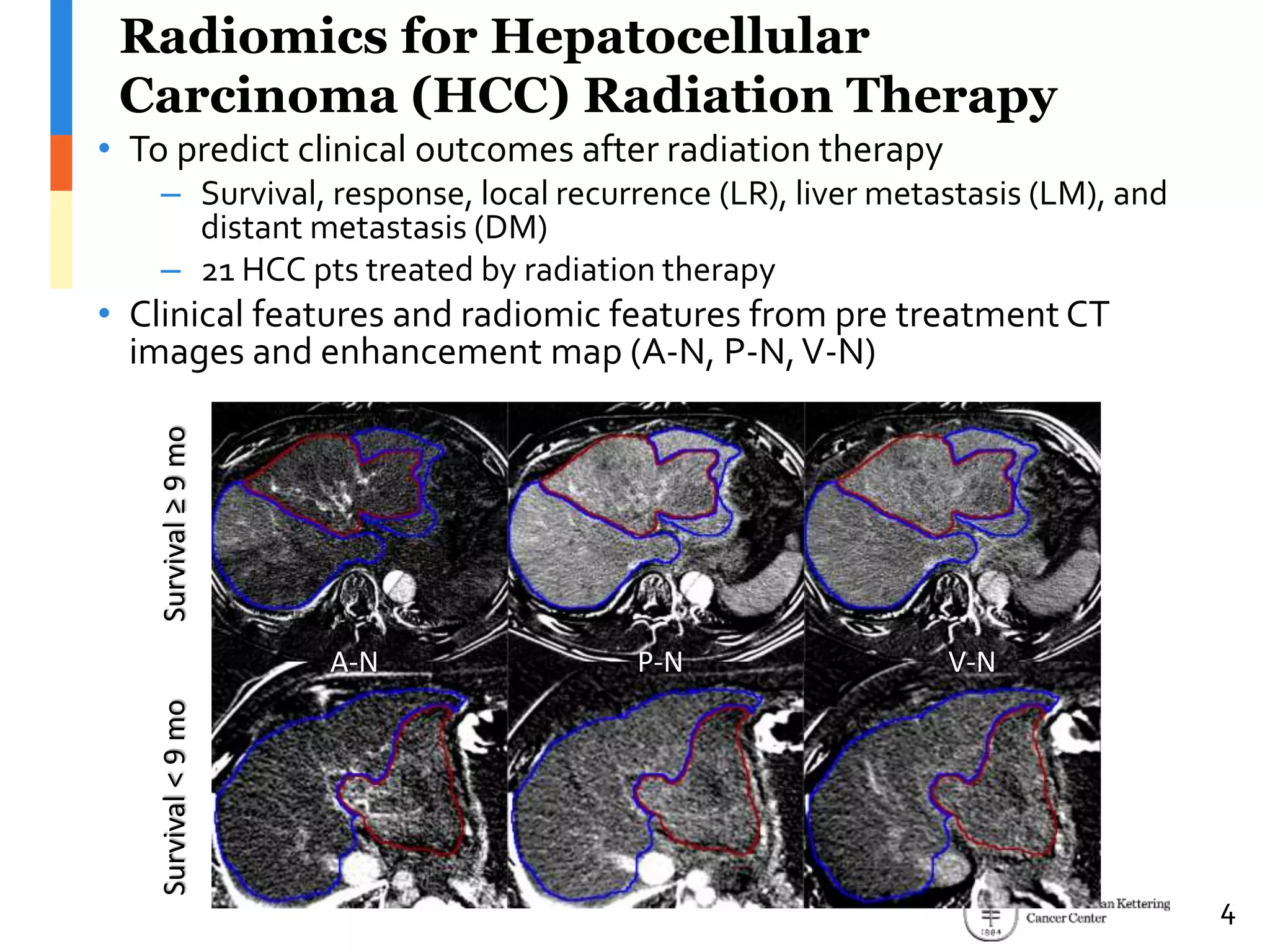
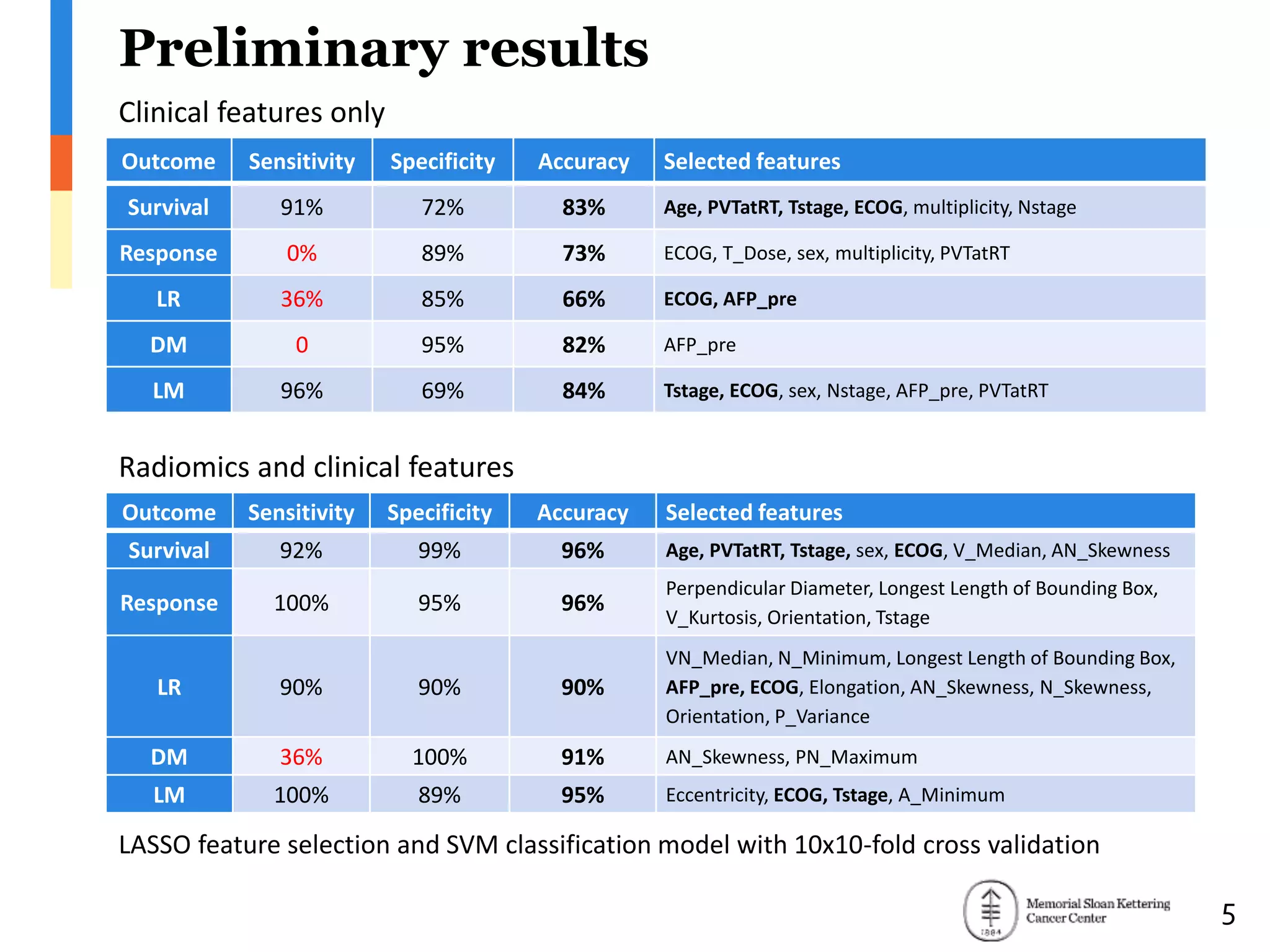
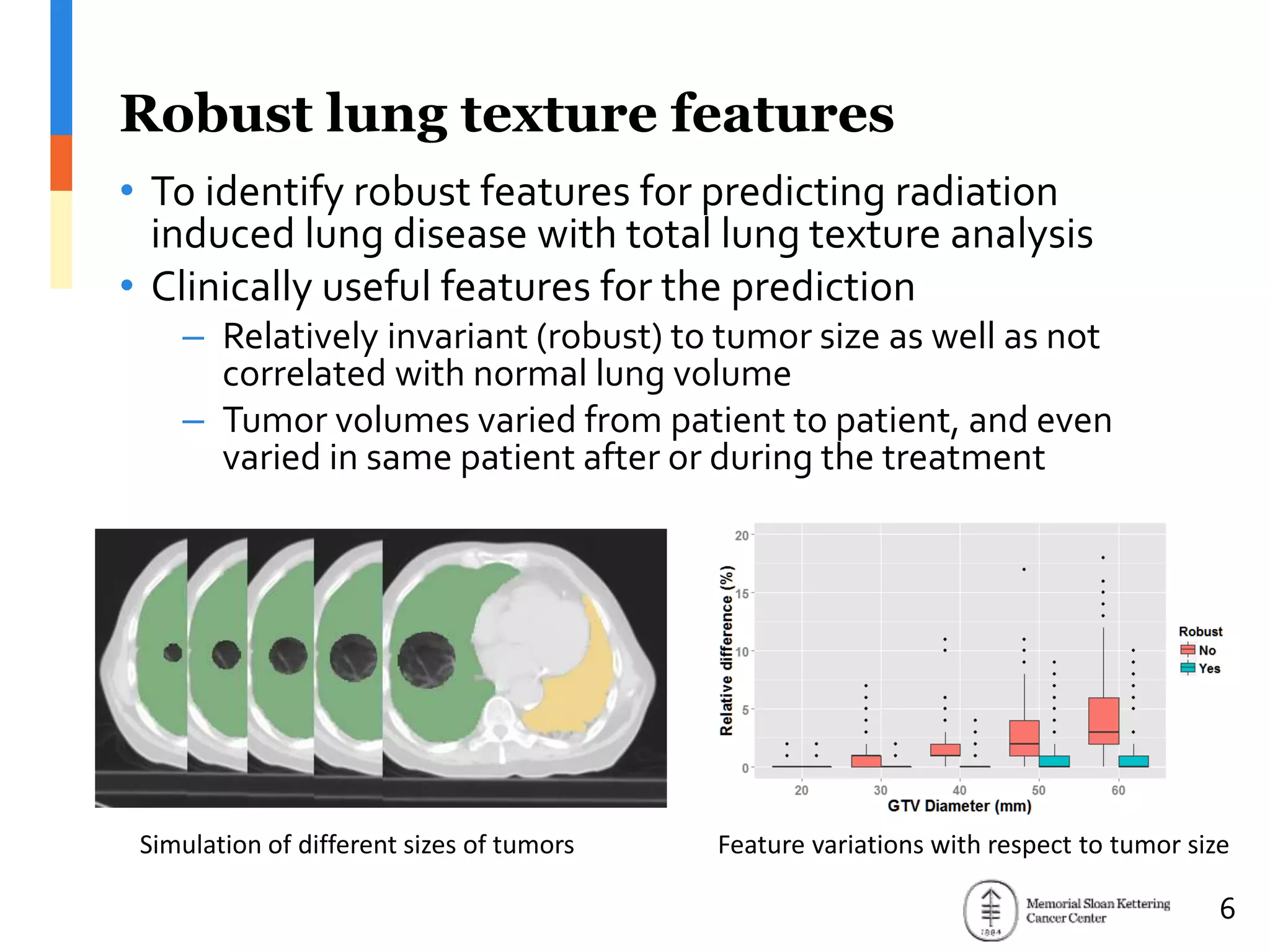
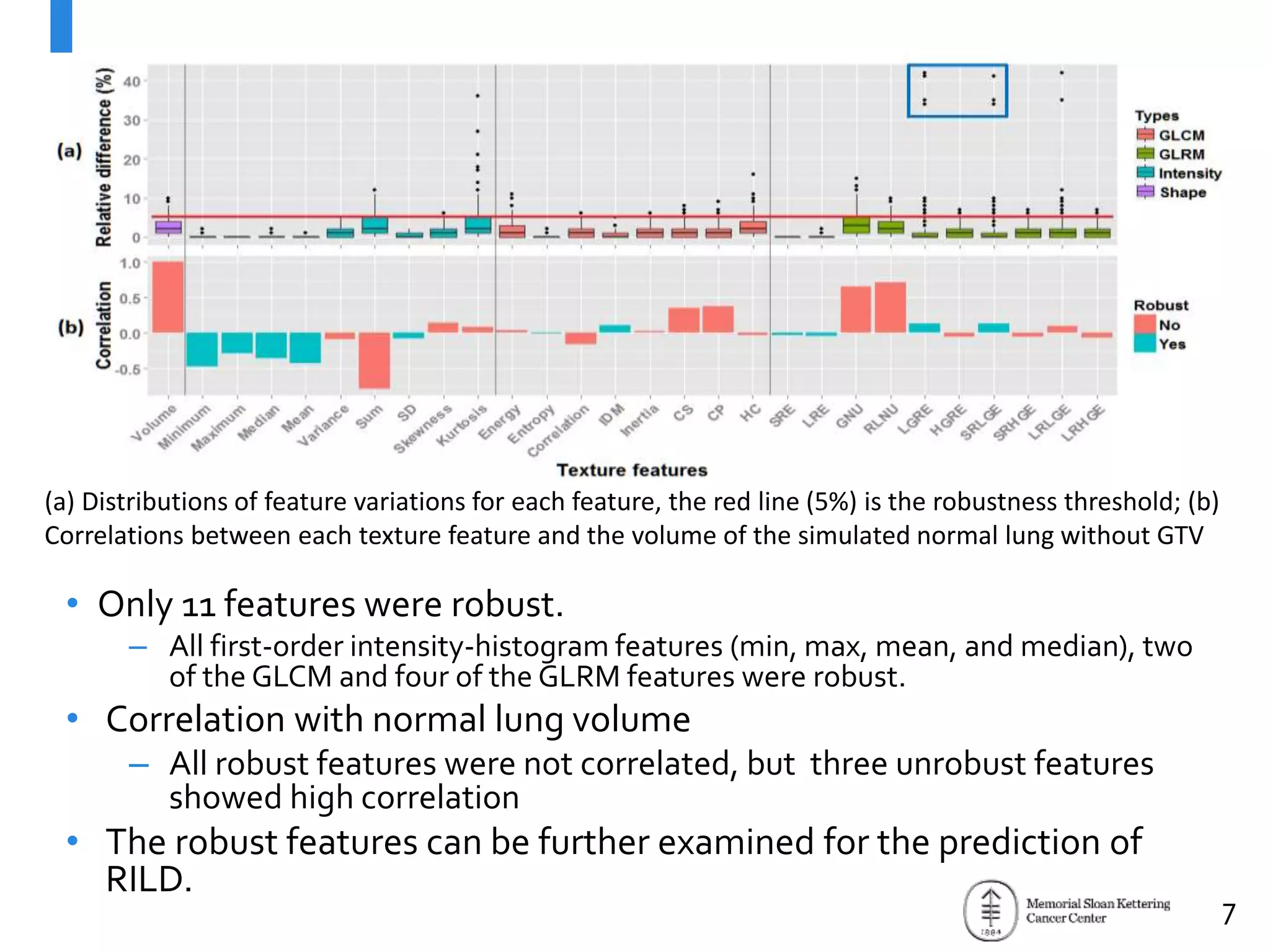
The document presents Wookjin Choi's research on medical image analysis, focusing on the automatic detection of pulmonary nodules using advanced algorithms and radiomics. It highlights the predictive capabilities of radiomic features in assessing malignancy and clinical outcomes in lung cancer and hepatocellular carcinoma, supported by extensive validation and feature selection techniques. Future work is outlined, emphasizing the development of new radiomic features and their integration with molecular biomarkers for improved lung screening.