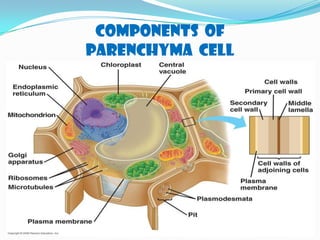
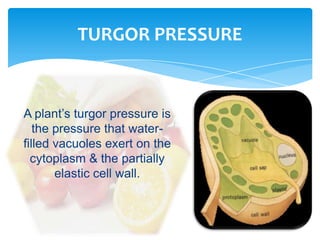
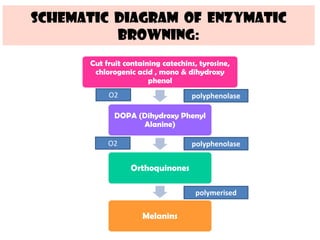
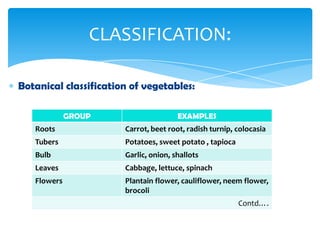
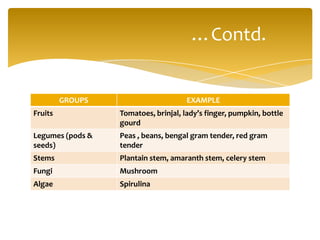
This document discusses the structure, composition and classification of fruits and vegetables. It begins by describing the simple and complex cell tissues that make up fruits and vegetables, including parenchyma, dermal, vascular, collenchyma and sclerenchyma tissues. It then examines the chemical composition of plant materials, listing the main components as carbohydrates, proteins, fats, vitamins, minerals, water and phytochemicals. The document proceeds to classify and describe different types of fruits and vegetables in detail. It explores the nutritional profiles, pigments, ripening processes, storage considerations and enzymatic and non-enzymatic browning reactions of fruits and vegetables.