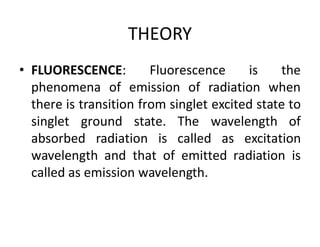
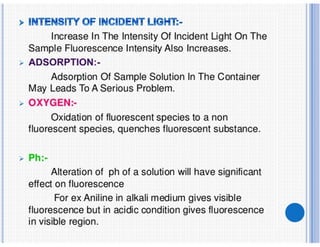
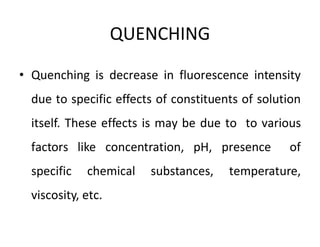
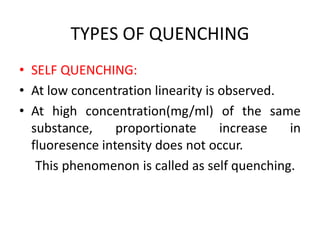
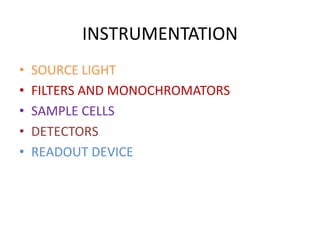
1) Spectrofluorimetry involves measuring the emission of radiation from a sample as electrons undergo transitions from an excited singlet state to a ground singlet state after absorbing UV/visible radiation.
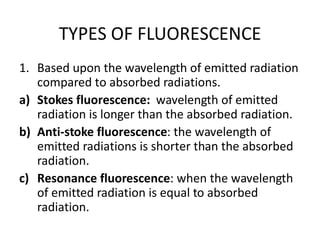
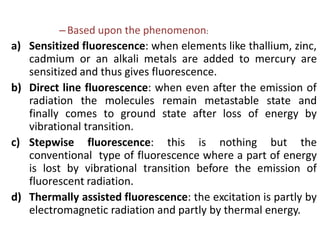
2) Samples can emit radiation through fluorescence or phosphorescence depending on whether the transition is from an excited singlet state or triplet state.
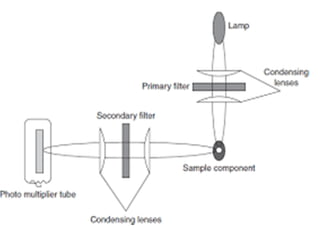
3) A double beam spectrofluorimeter uses excitation and emission monochromators to select wavelengths and a photomultiplier tube detector to measure the emitted radiation from a sample, providing a quantitative analysis of fluorescent compounds.