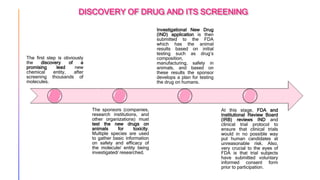
The drug development and review process involves several stages. Drugs are first tested on animals to assess safety before human clinical trials. Clinical trials involve 3 phases testing on increasingly large groups of human subjects to evaluate safety, efficacy, and optimal dosage. If results are promising, drug sponsors submit a New Drug Application to the FDA including all trial data. The FDA thoroughly reviews the application over 6-10 months before approving the drug or requesting more information. Post-approval, the FDA continues monitoring the drug for safety issues and requires further studies if needed.