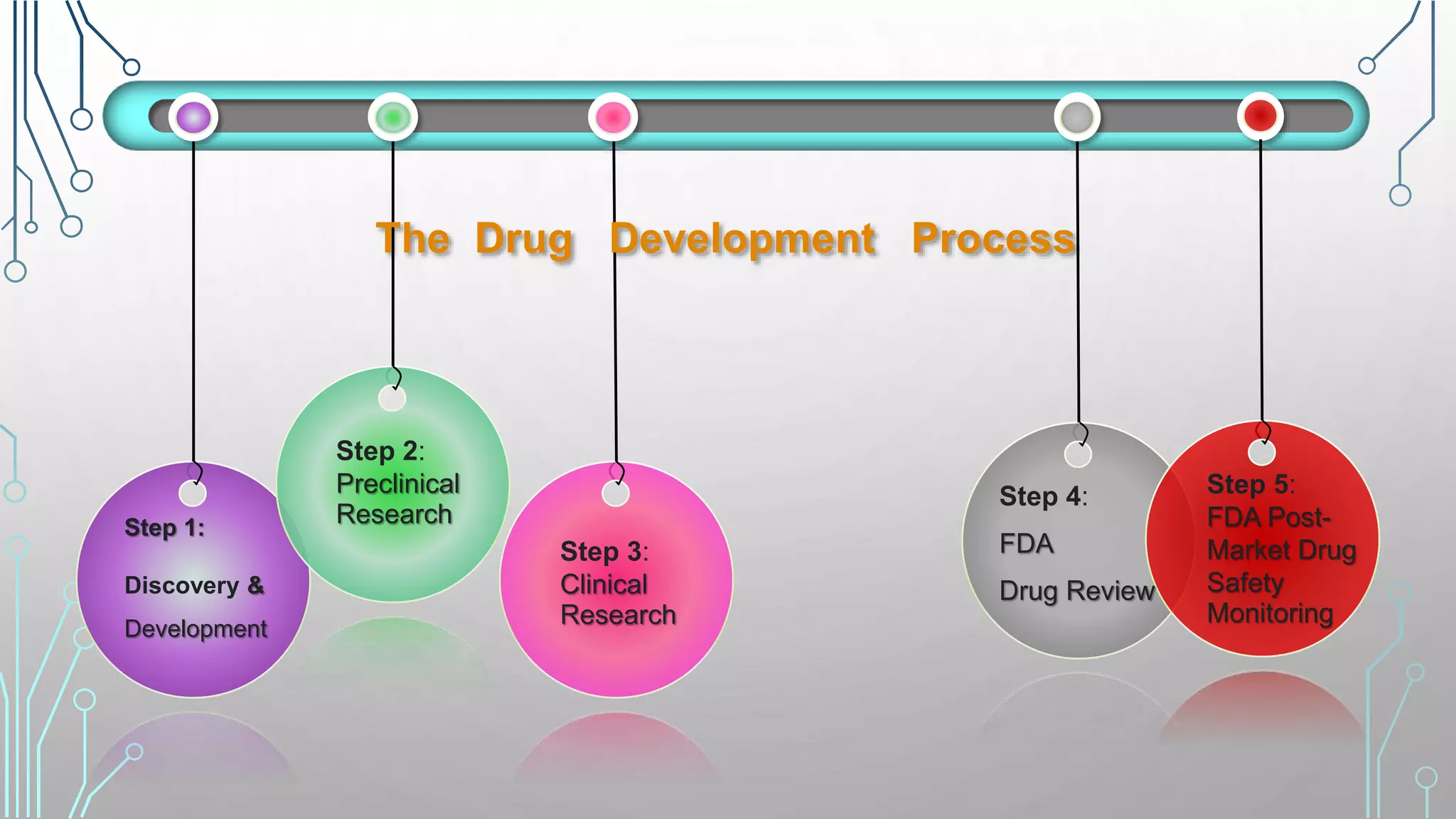
The drug development process involves 5 main steps: (1) discovery and development through preclinical research on animals, (2) clinical research conducted in 4 phases with human subjects to test safety, efficacy, and side effects, (3) FDA drug review of the New Drug Application and clinical trial data, (4) potential FDA approval or denial, and (5) ongoing FDA post-market drug safety monitoring through manufacturer inspections, surveillance of large electronic health databases, and analysis of reported adverse events. The overall process aims to discover new drugs, understand how they work, ensure they are safe and effective for their intended use through clinical trials, and continuously monitor approved drugs for unexpected safety issues.