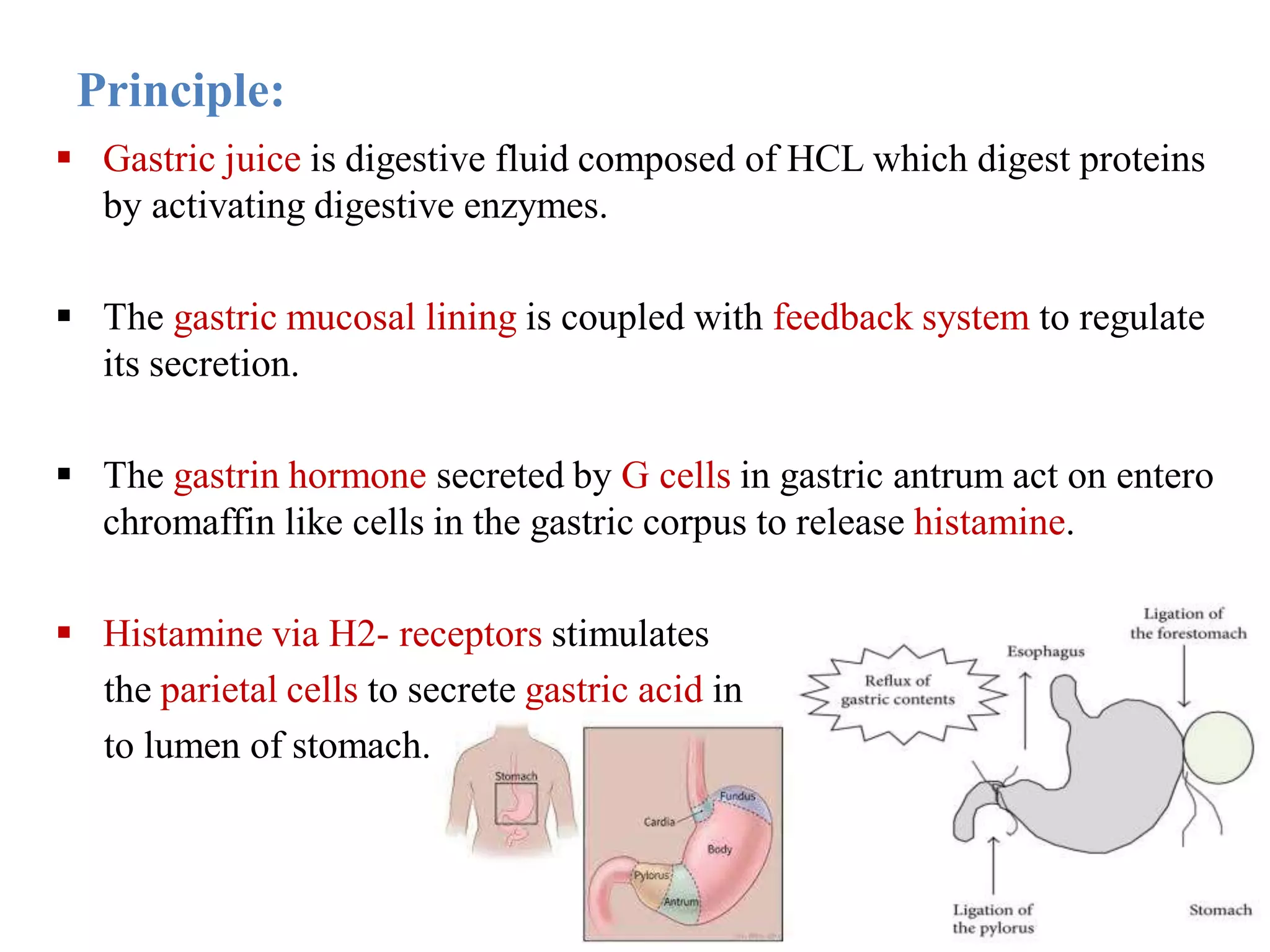
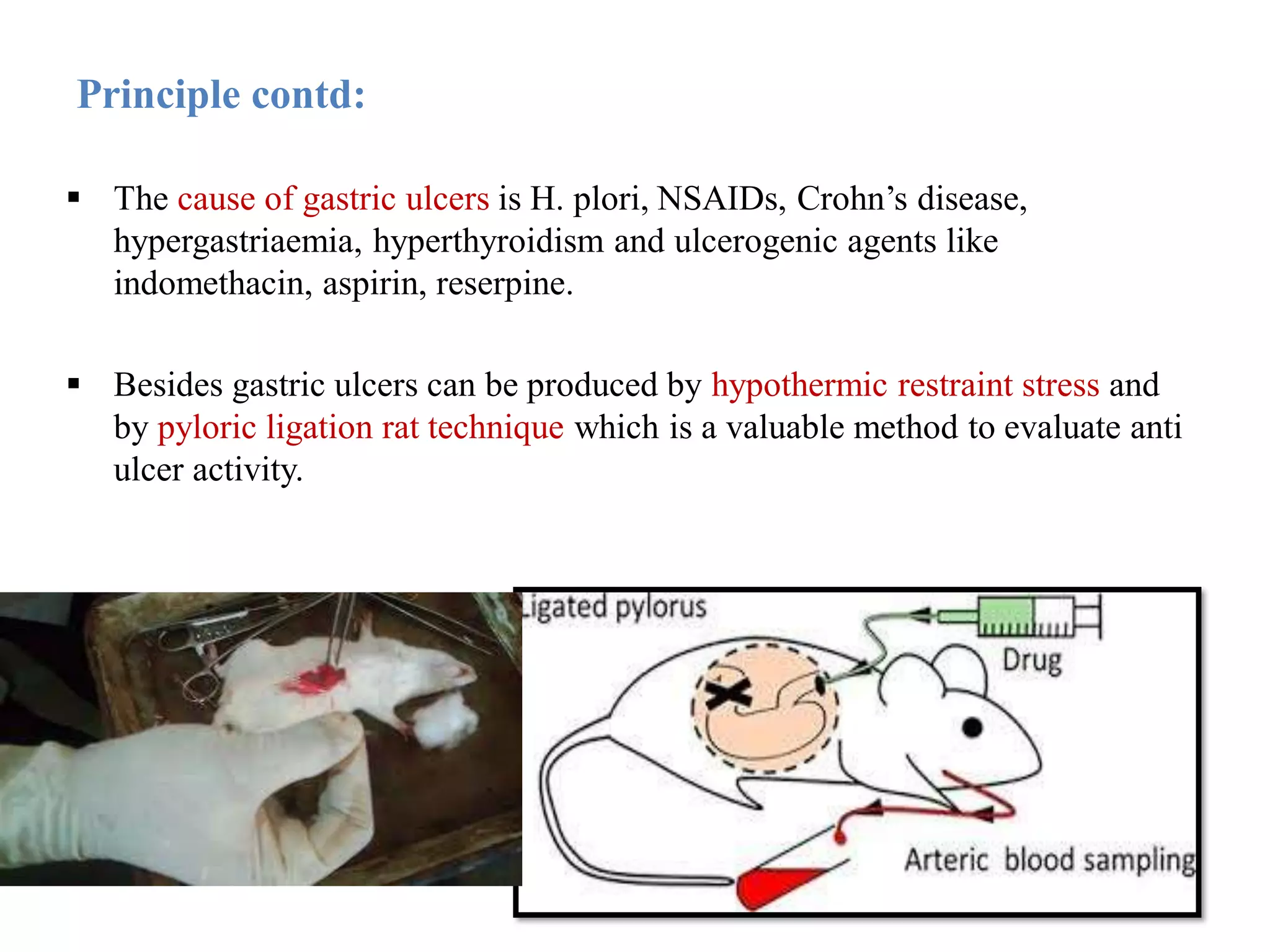
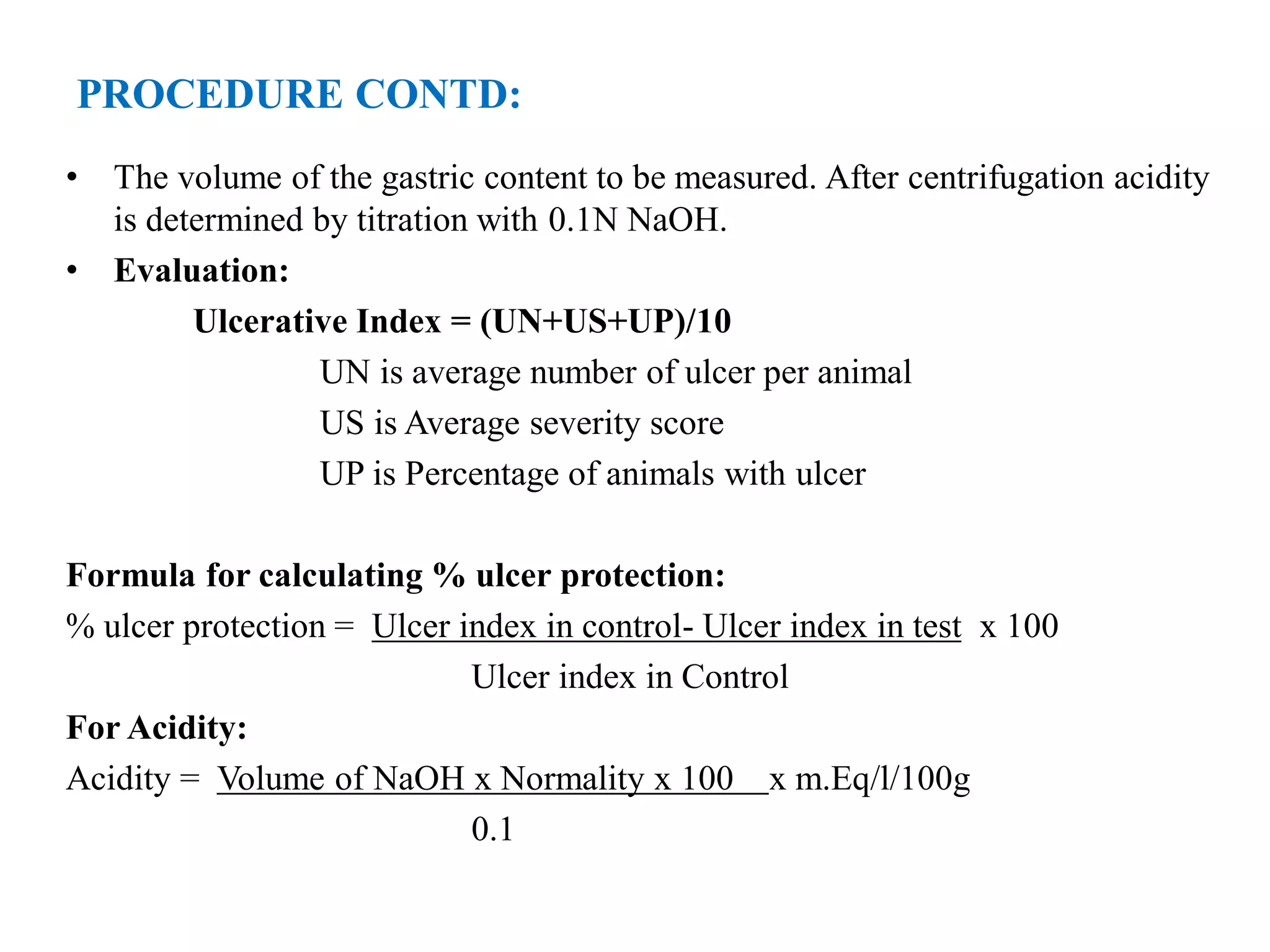
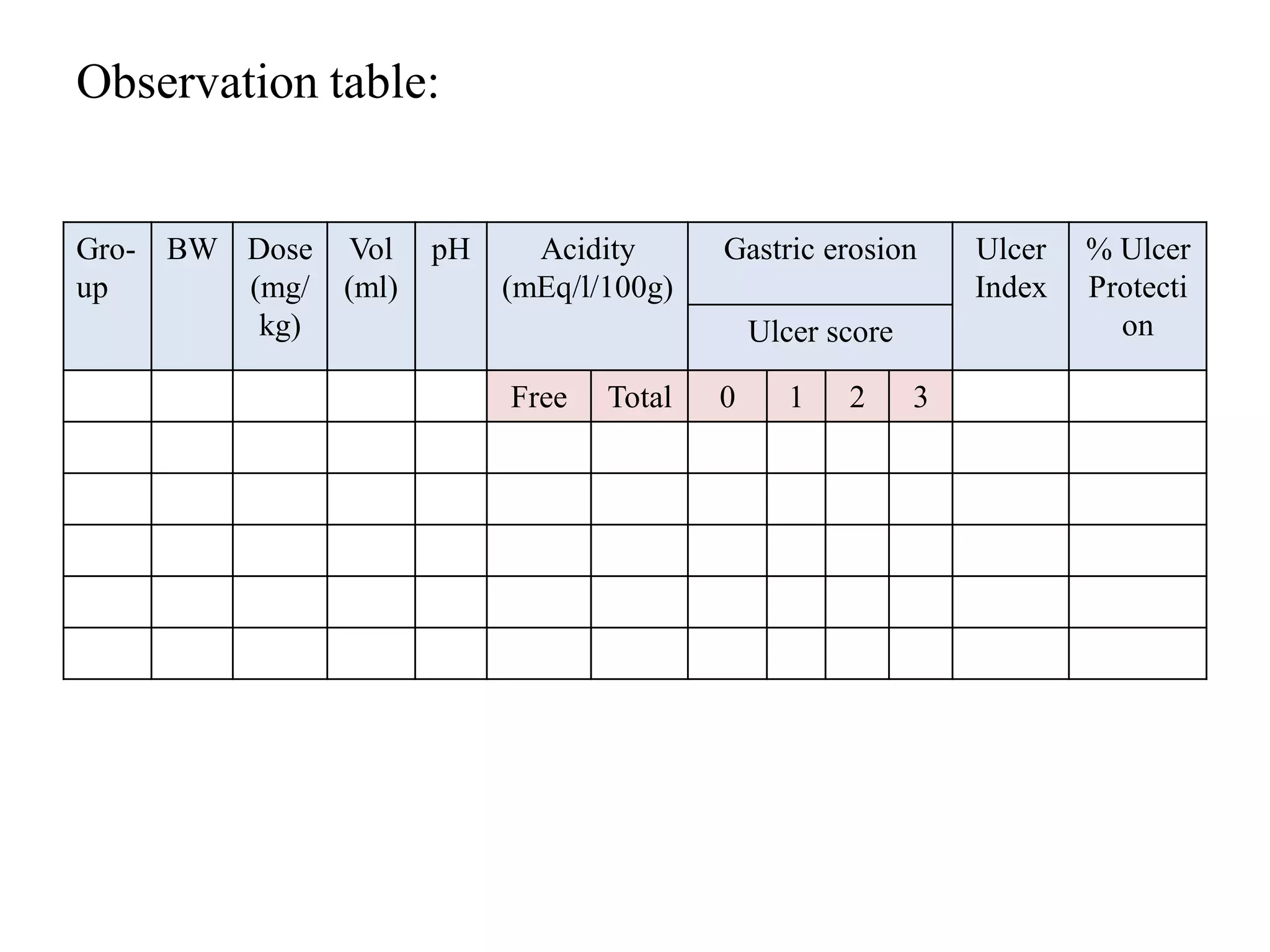
This document describes a study to evaluate the anti-ulcer activity of a drug using a pyloric ligation rat model. It outlines the requirements including animal type, drugs to be tested, and equipment needed. It then describes the principle behind gastric acid secretion and ulcer formation. The procedure involves starving rats for 24 hours, ligating the pylorus, administering test compounds, sacrificing the rats, and examining the stomachs to measure ulcer indices, gastric acidity, and percent ulcer protection. Treatment with the standard drug ranitidine is expected to reduce ulceration, acidity, and increase ulcer protection compared to saline control.