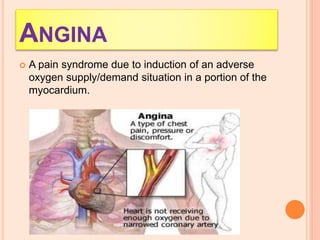
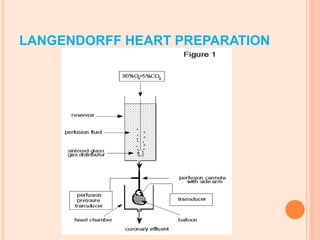
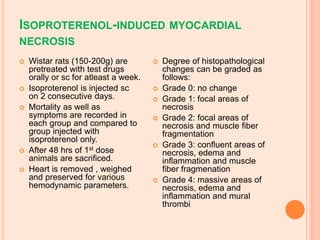
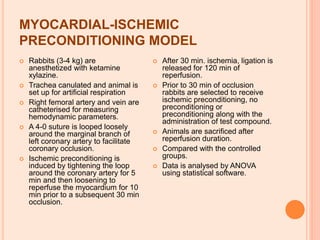
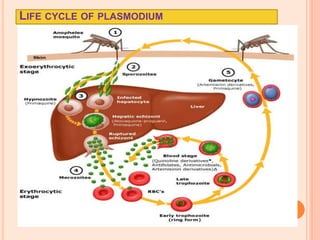
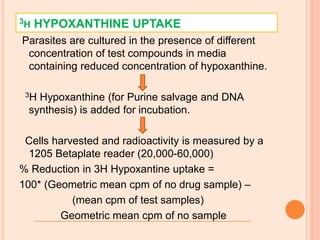
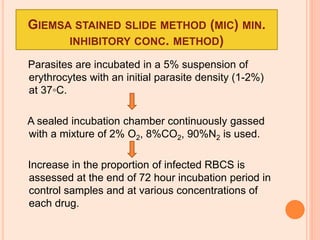
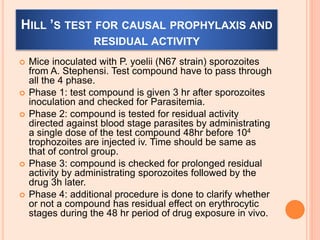
The seminar presented by Charu Pundir focuses on the evaluation of antianginal and antimalarial drugs through various in vitro and in vivo models. It elaborates on antianginal models, types of angina, associated treatments, and detailed methodologies for testing drug efficacy using heart and rat models. Additionally, antimalarial drug screening methods and treatments for malaria caused by Plasmodium species are discussed, citing references for further reading.