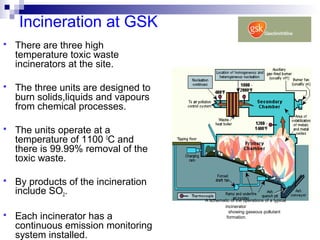
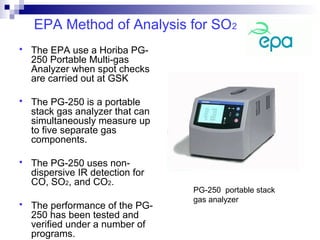
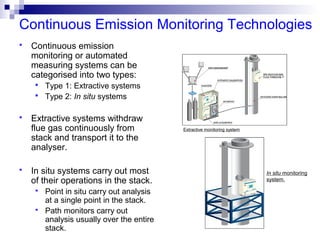
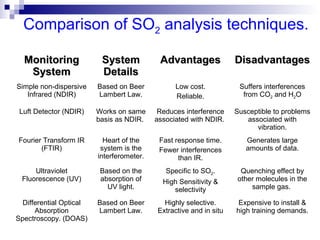
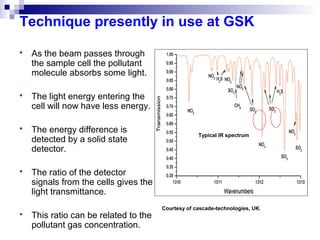
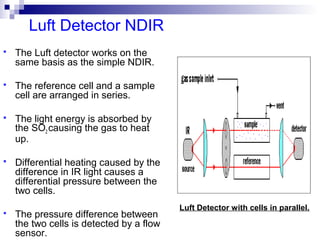
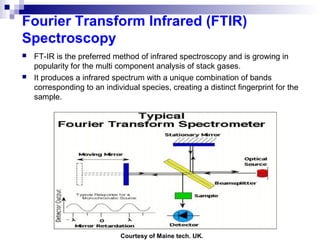
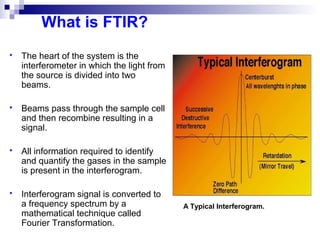
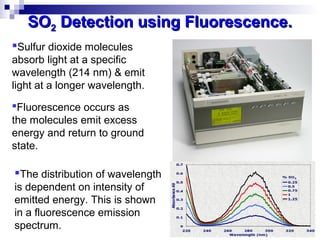
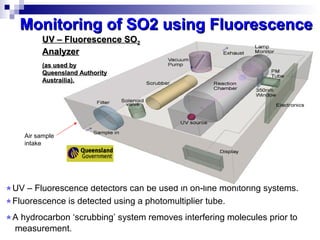
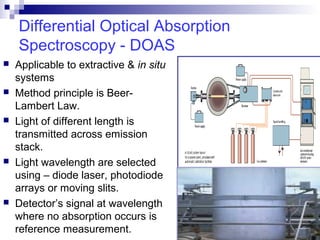
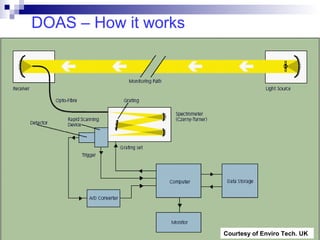
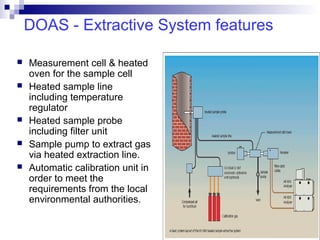
This document discusses air emissions monitoring methods for sulfur dioxide (SO2) at GlaxoSmithKline (GSK) in Ireland. It provides an overview of SO2 monitoring requirements and techniques. GSK currently uses non-dispersive infrared spectroscopy to continuously monitor SO2 emissions in compliance with EPA standards. Alternative techniques like fluorescence analysis and differential optical absorption spectroscopy are also described that could provide more sensitive or selective SO2 detection. In conclusion, GSK is meeting all regulatory standards but may consider updating methods in the future if standards change.