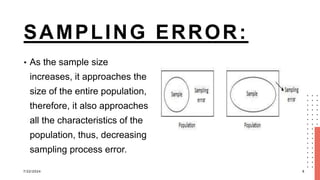
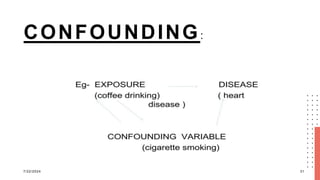
The document discusses errors and biases in research, highlighting that errors can be random or systematic and that biases can occur at any stage of research, impacting the validity of findings. It details various types of errors, such as sampling error and Type I and II errors, as well as biases like selection bias and information bias, and their effects on study outcomes. The conclusion emphasizes the importance of recognizing and addressing these issues to maintain the quality and trustworthiness of research data.