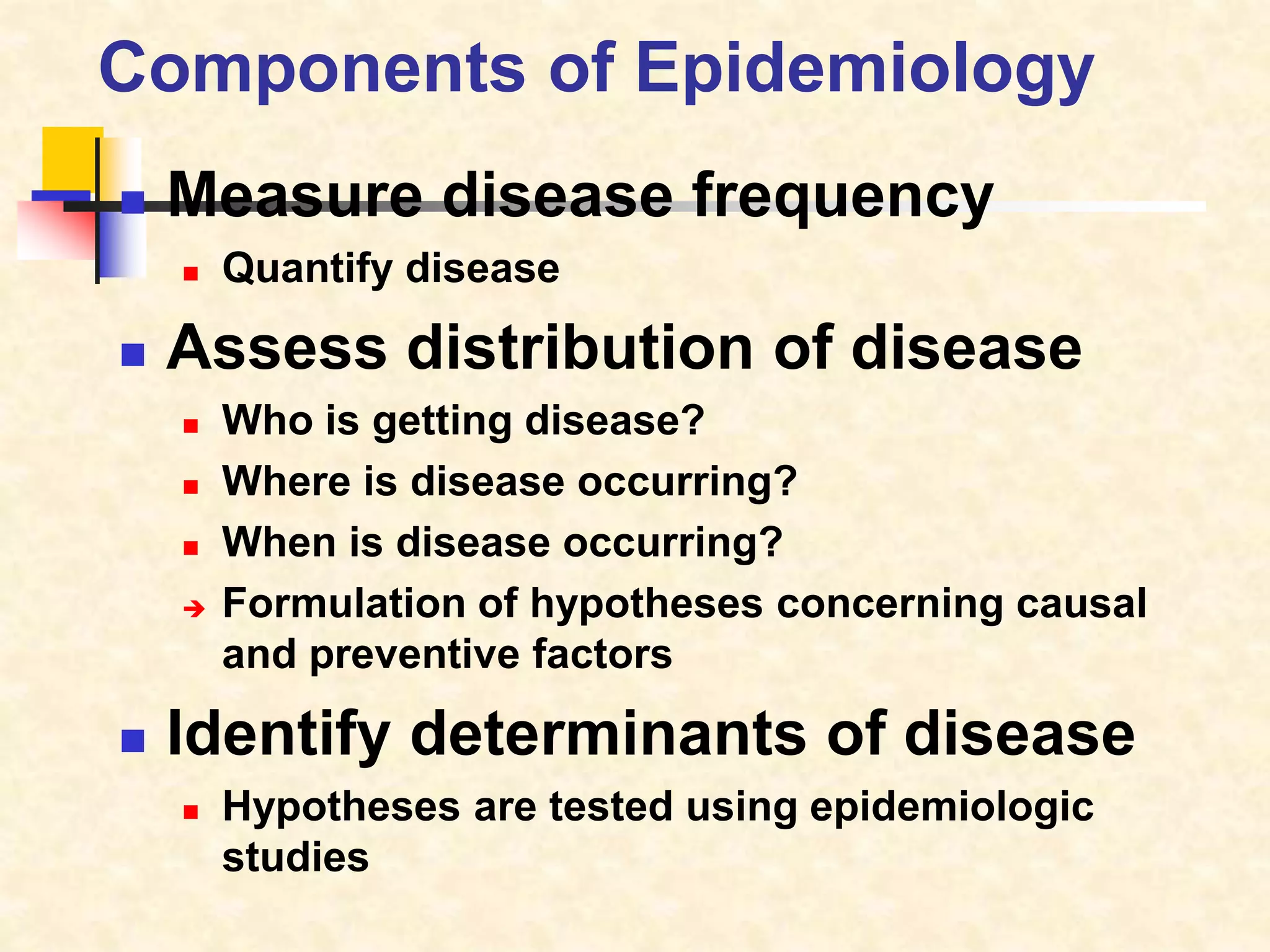
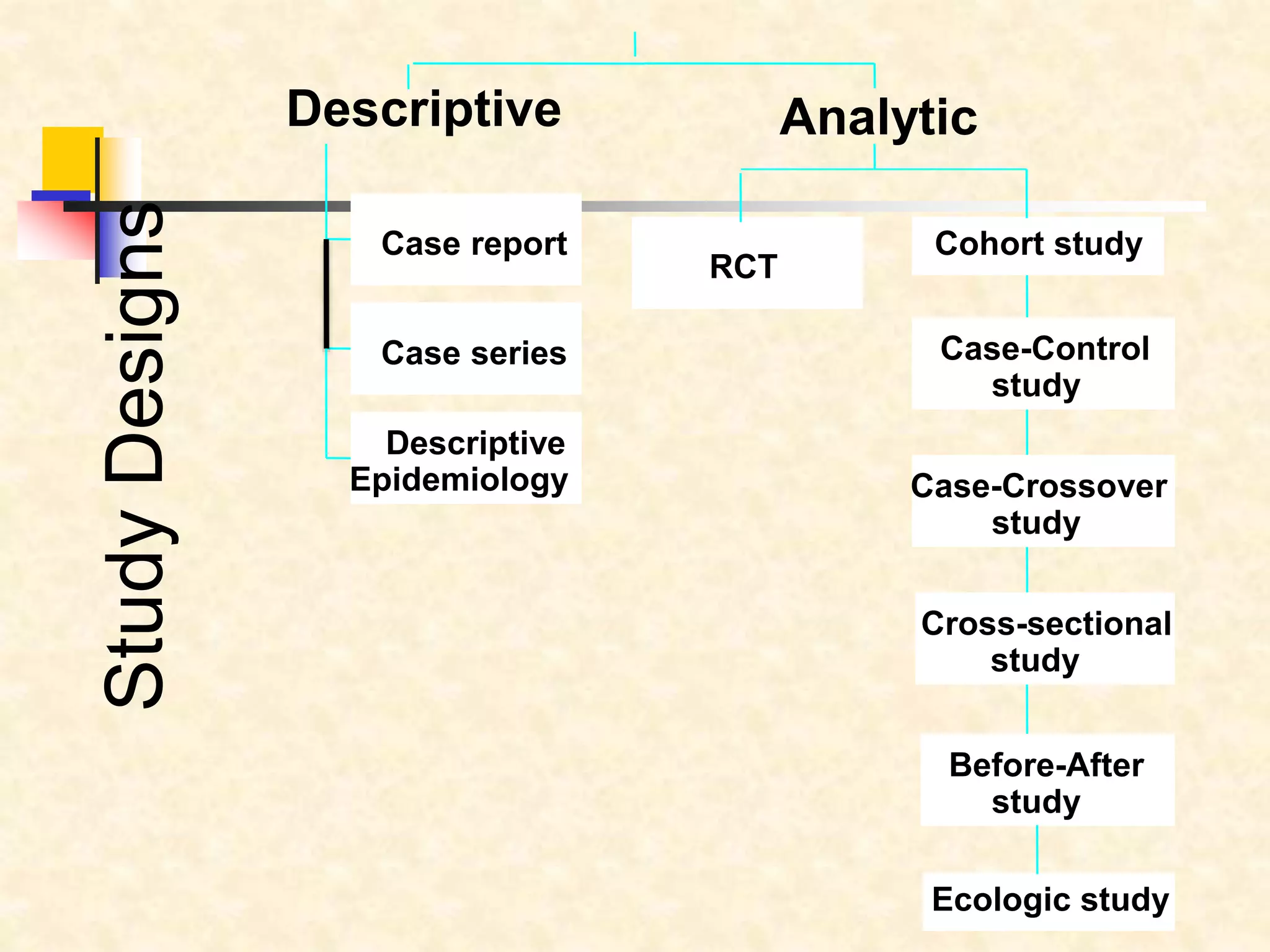
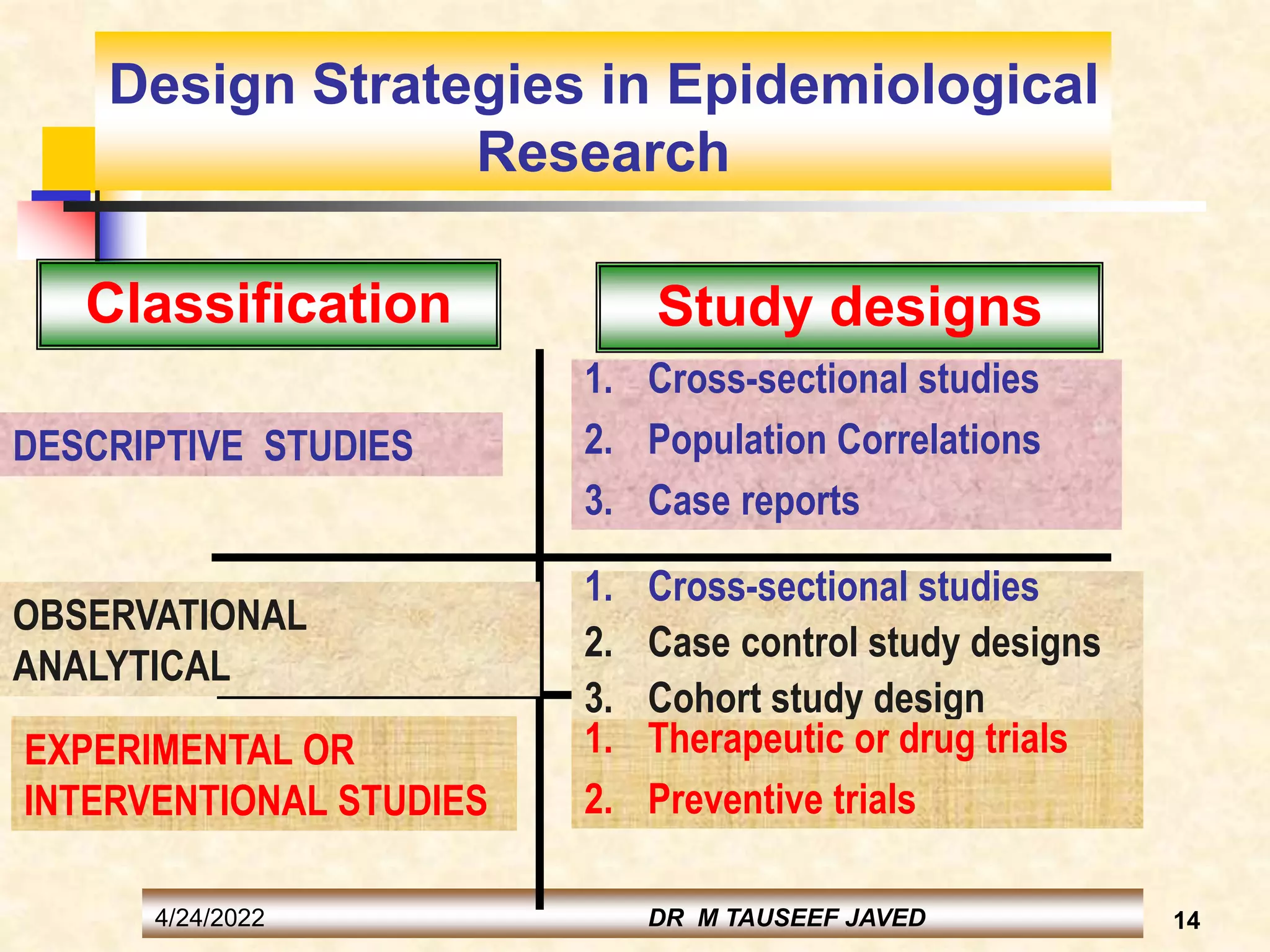
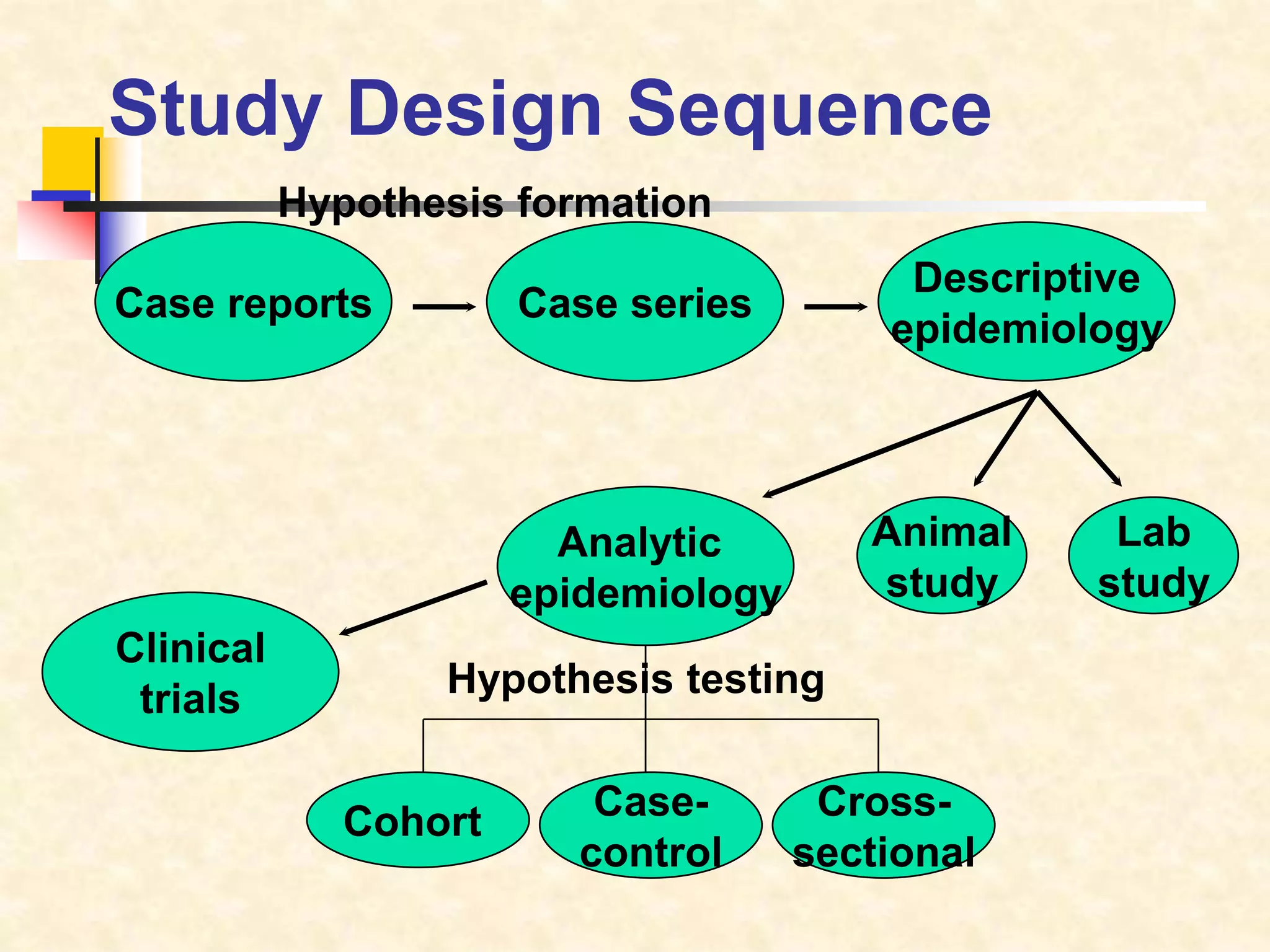
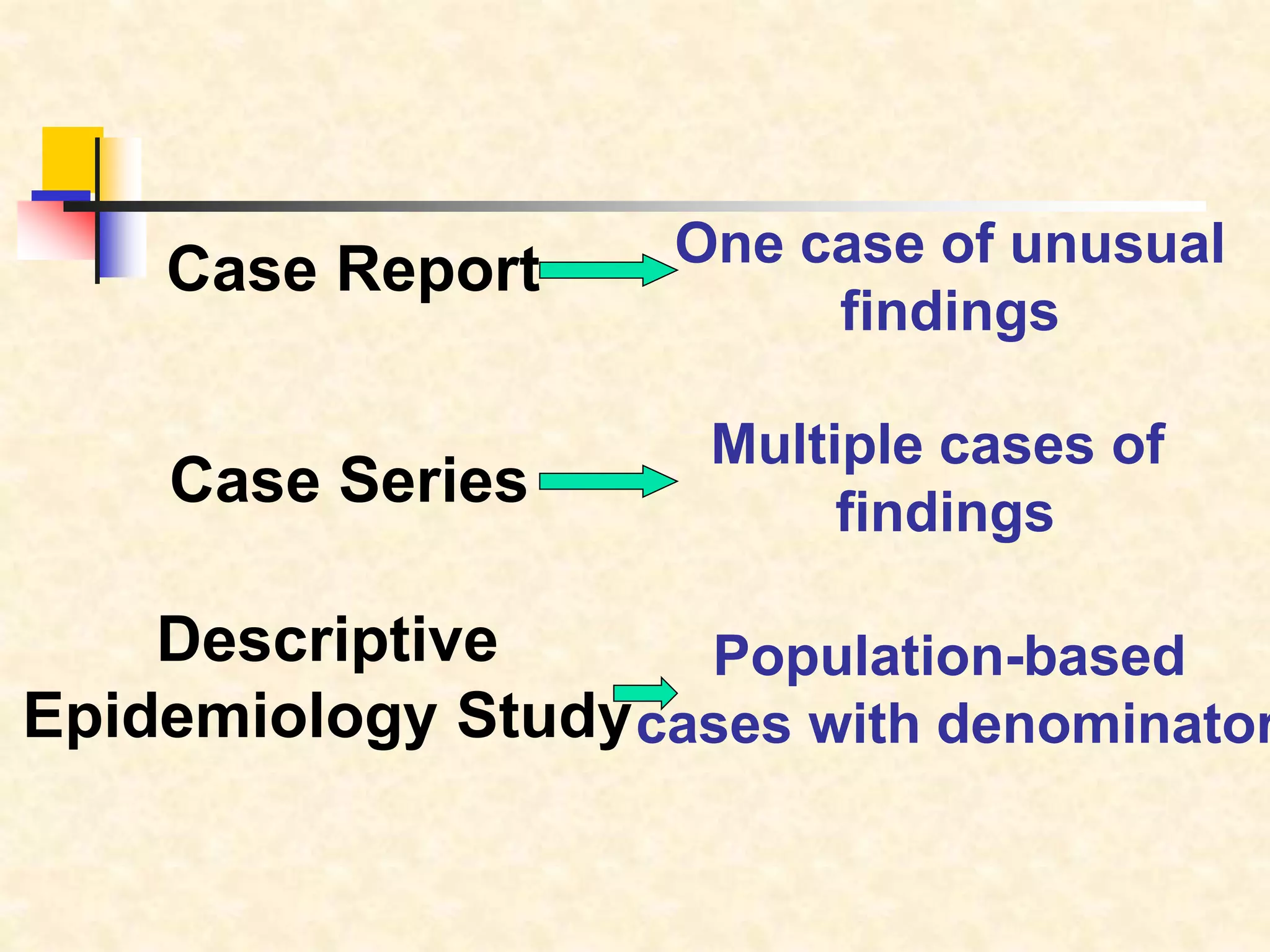
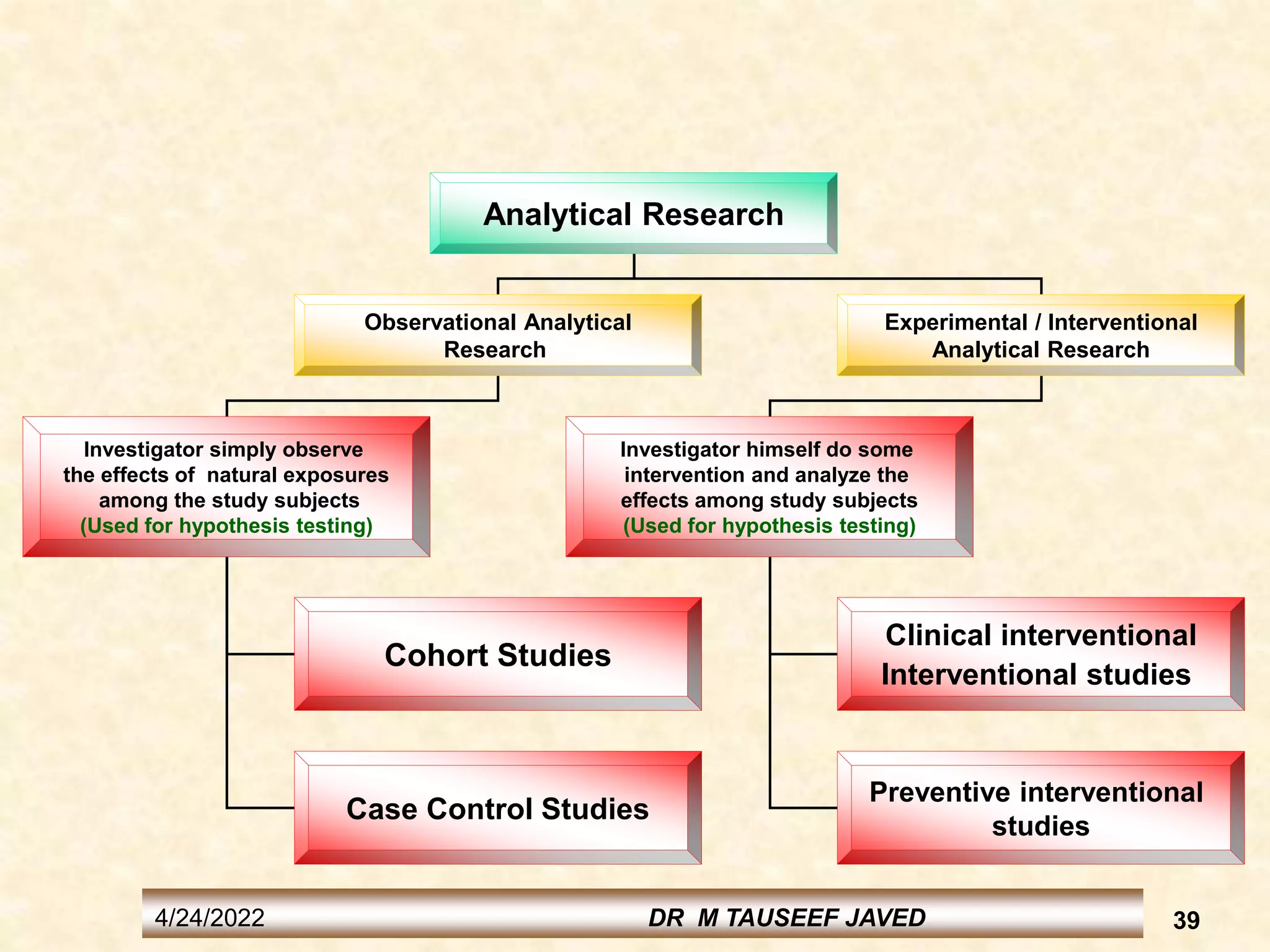
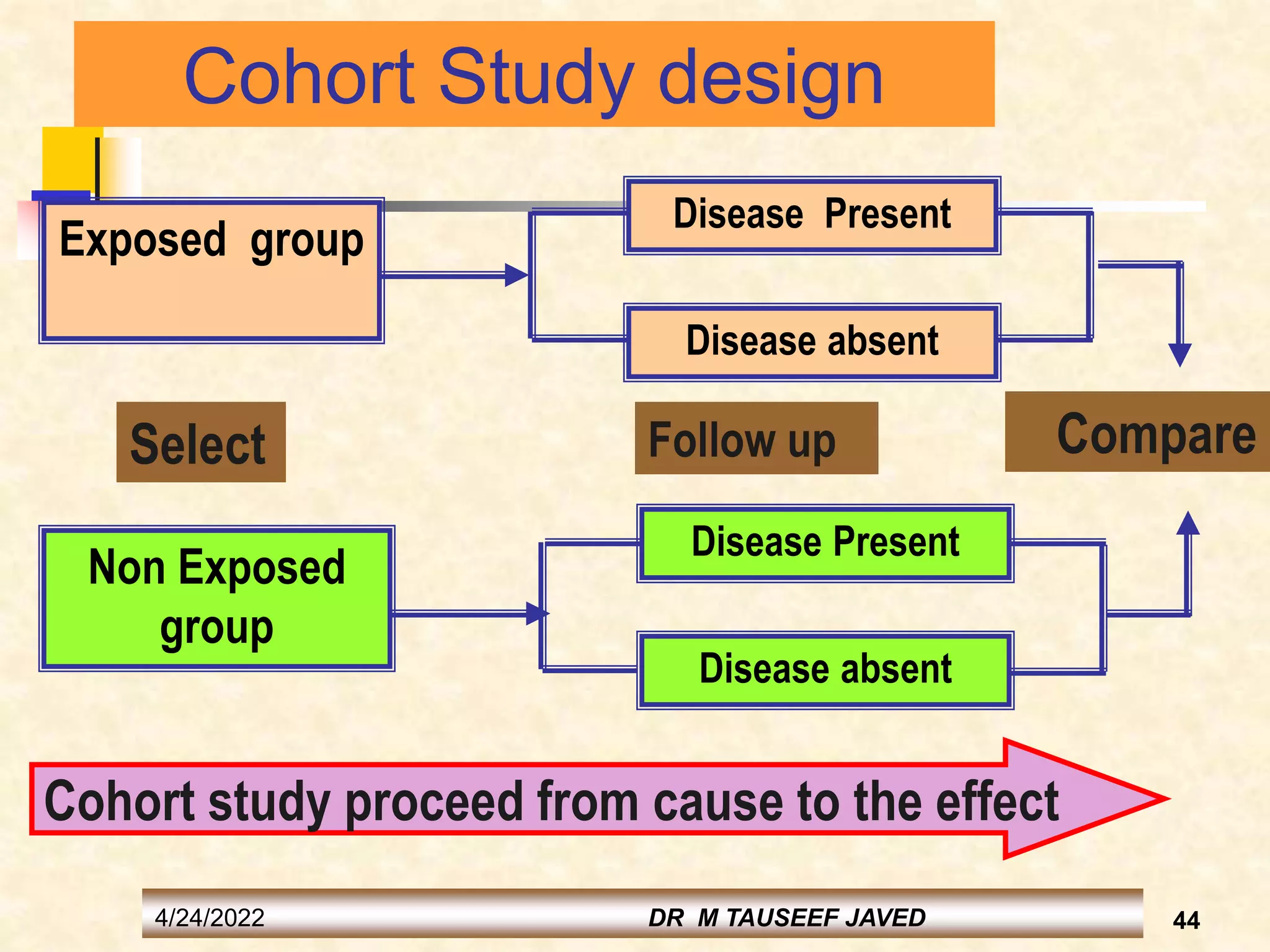
The document provides information on various study designs used in epidemiology, including descriptive and analytical studies. Descriptive studies like case reports and case series are used to identify frequencies, distributions and generate hypotheses. Analytical observational studies include cross-sectional, cohort and case-control studies used to test hypotheses. Cohort studies specifically follow groups over time to study exposure-disease relationships. They involve selecting exposed and non-exposed groups, defining and measuring exposures, following up to assess outcomes, and analyzing results including relative risk calculations.