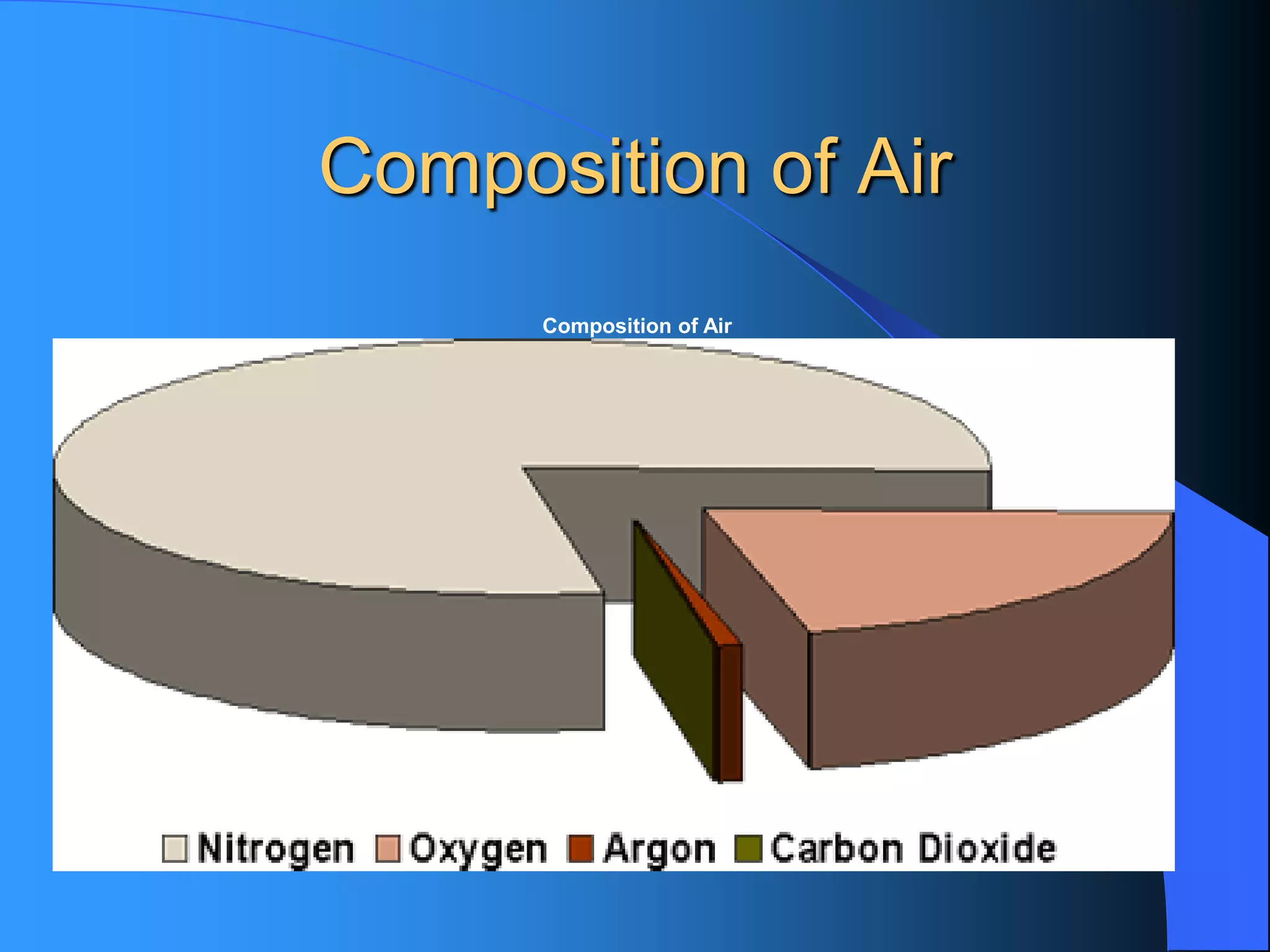
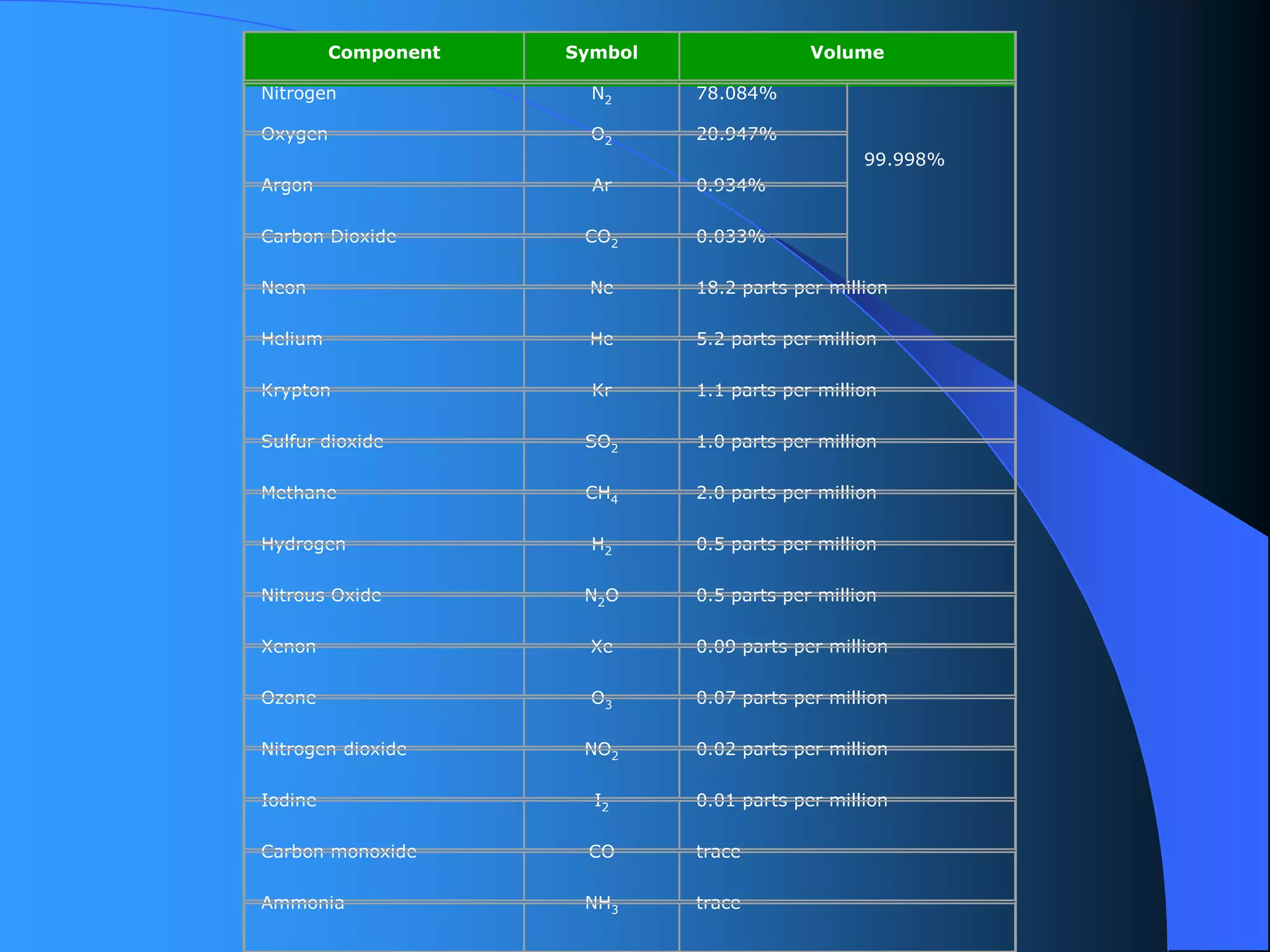
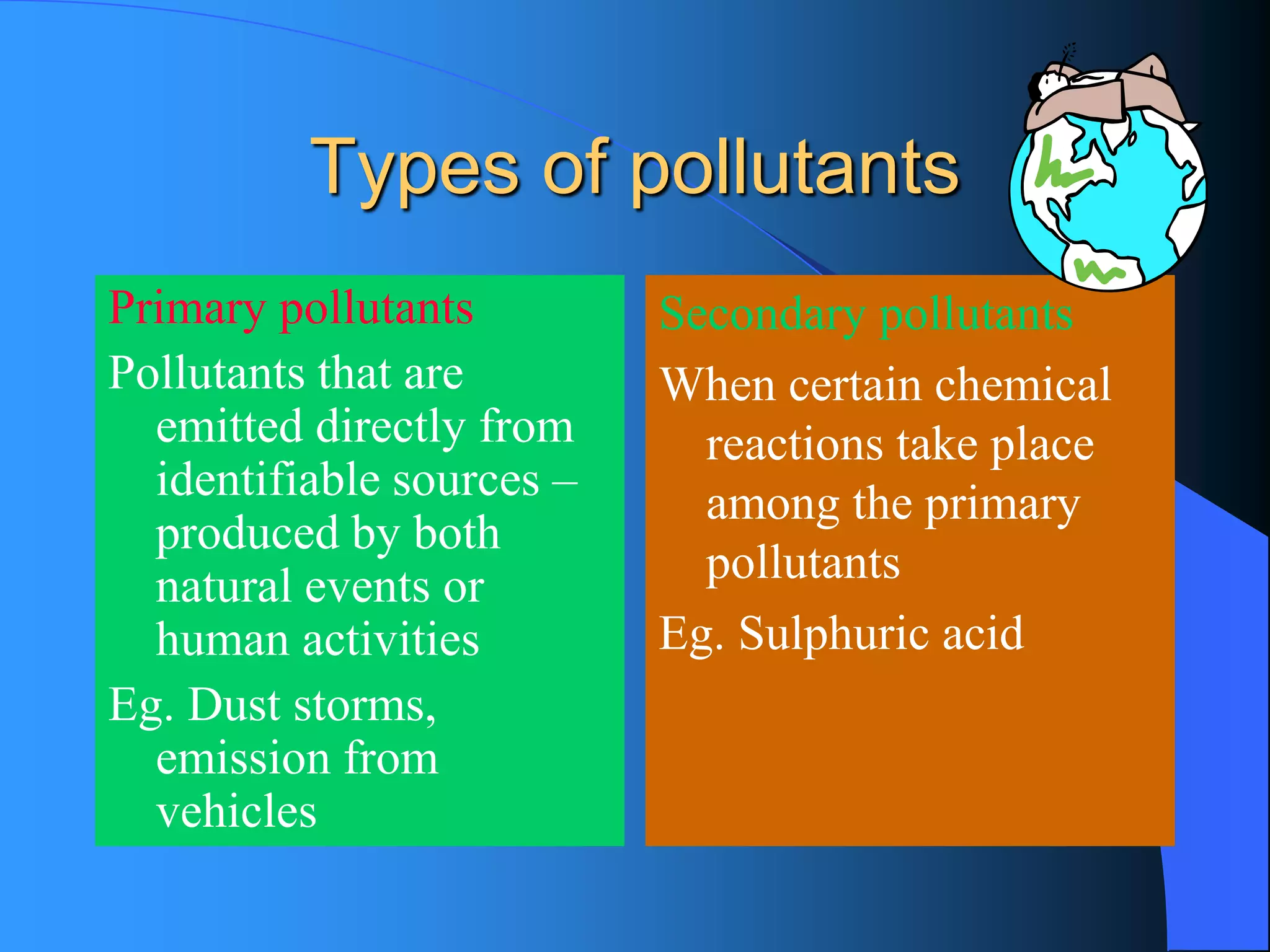
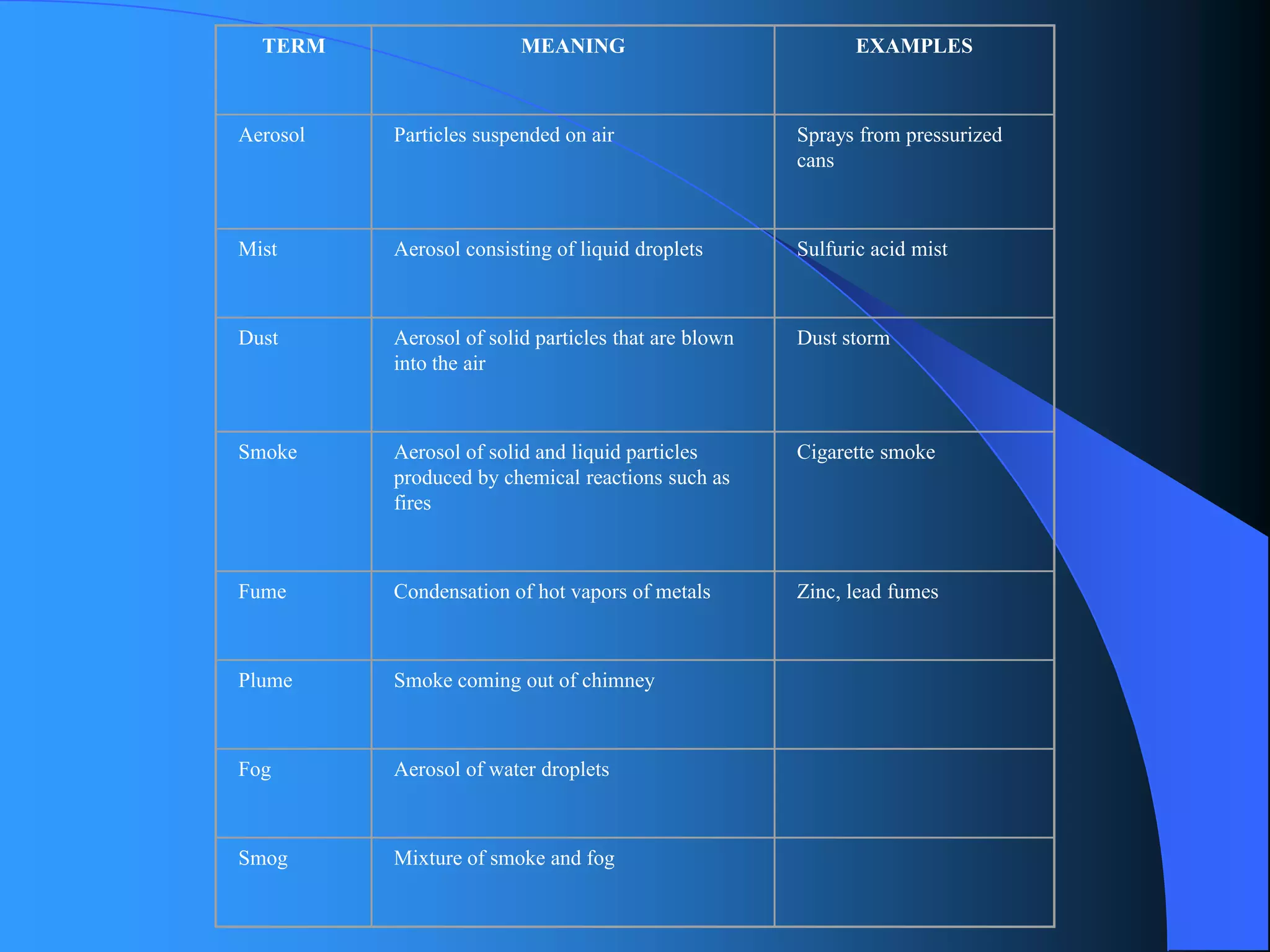
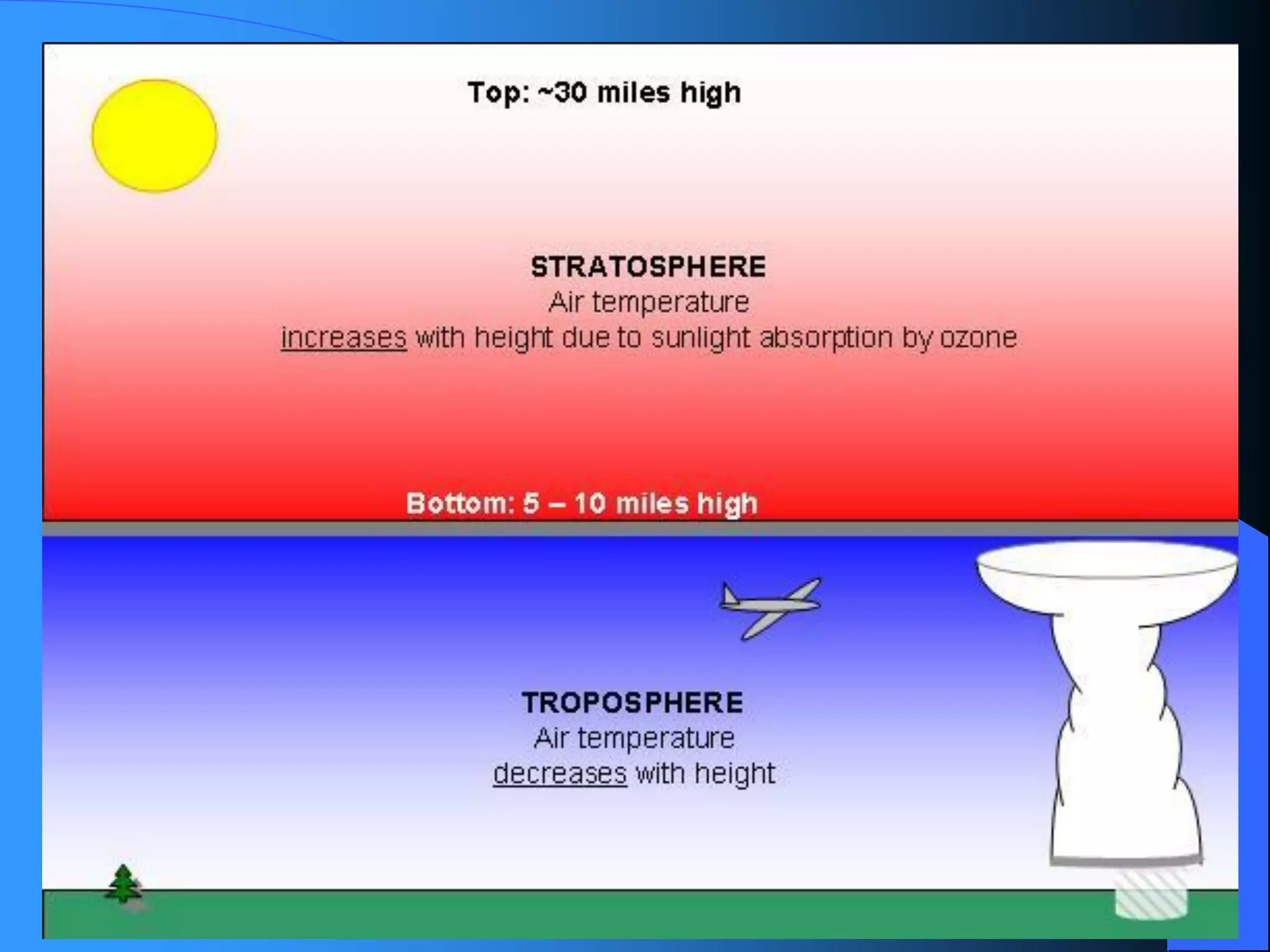
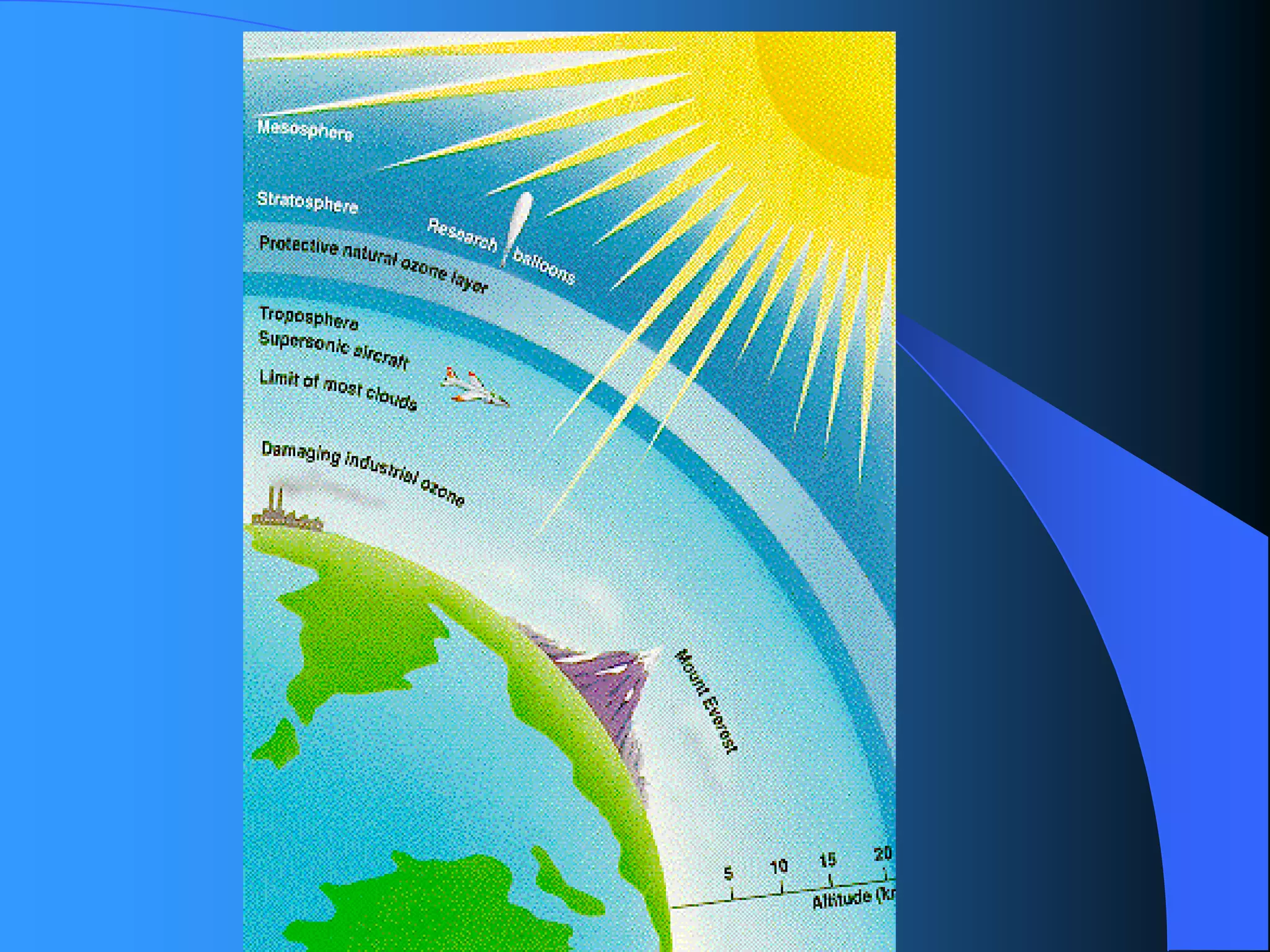
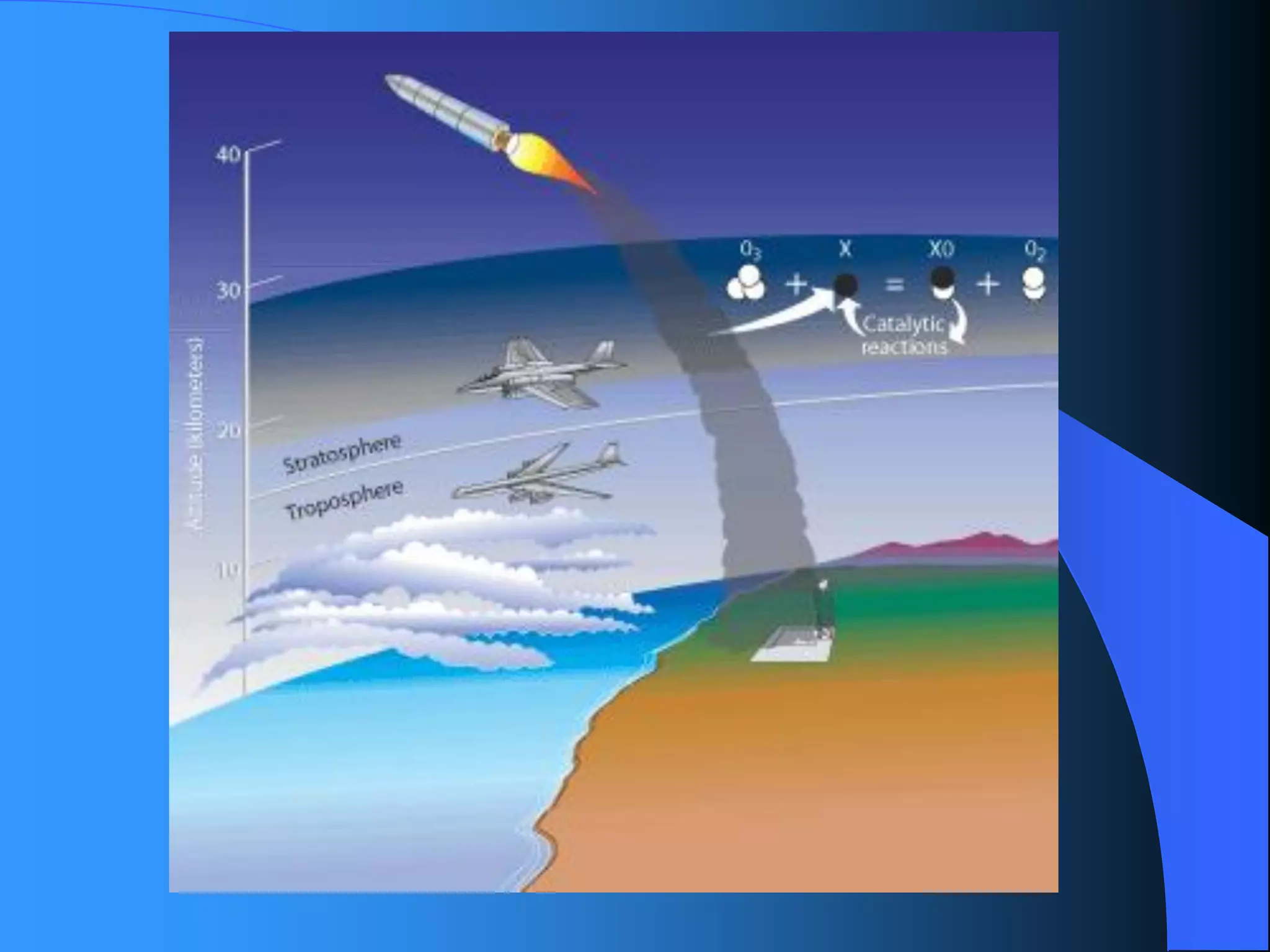
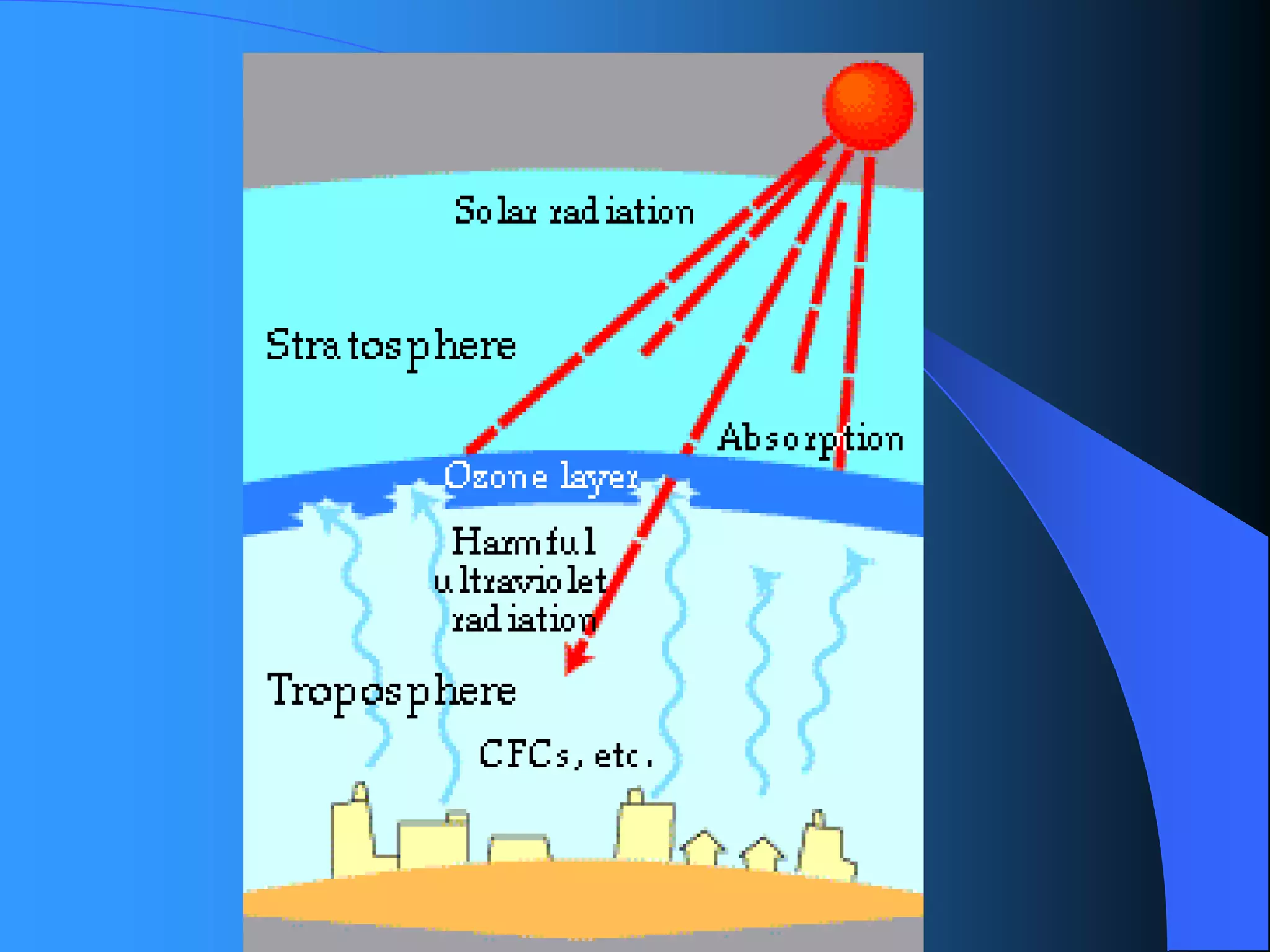
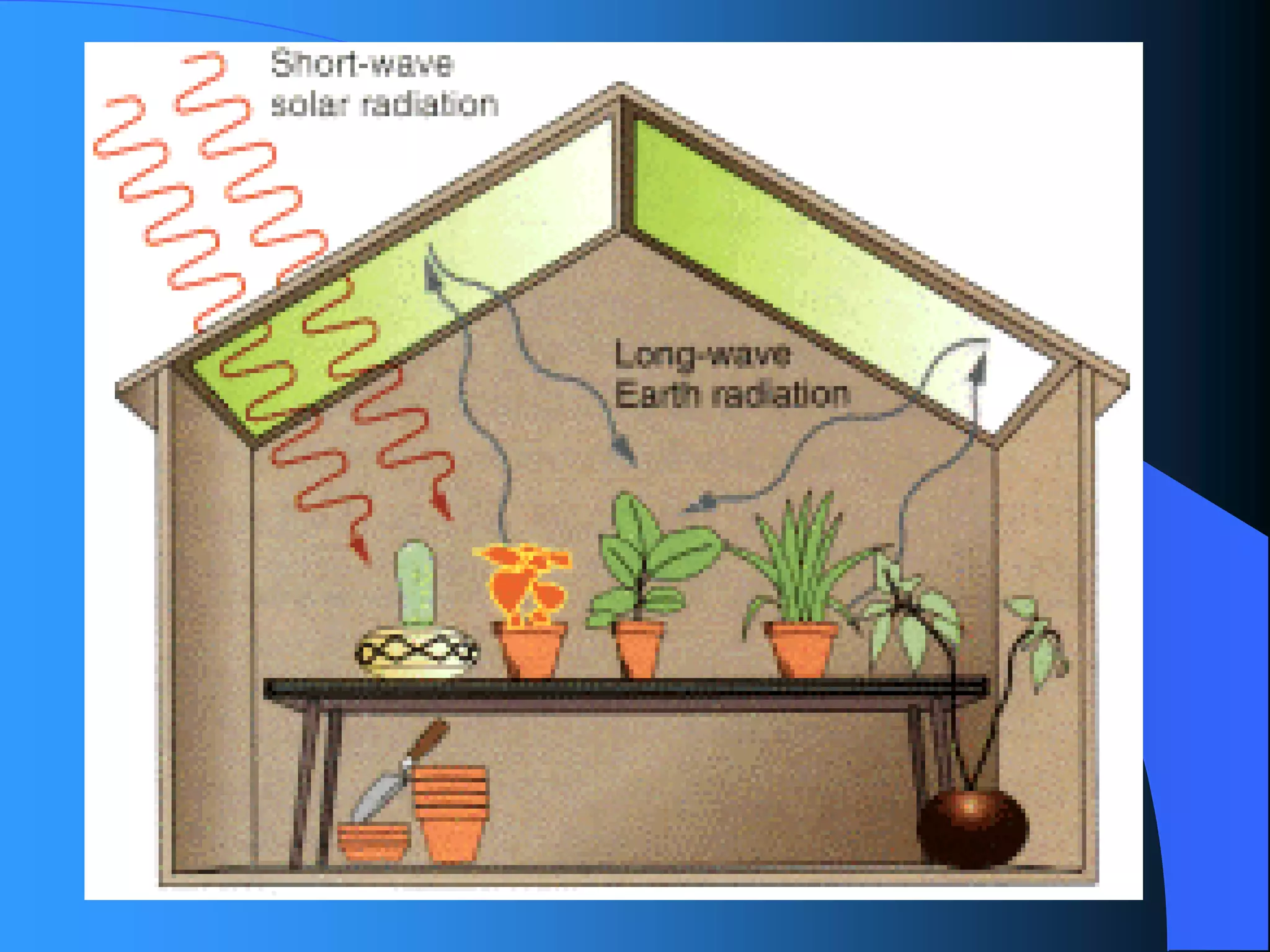
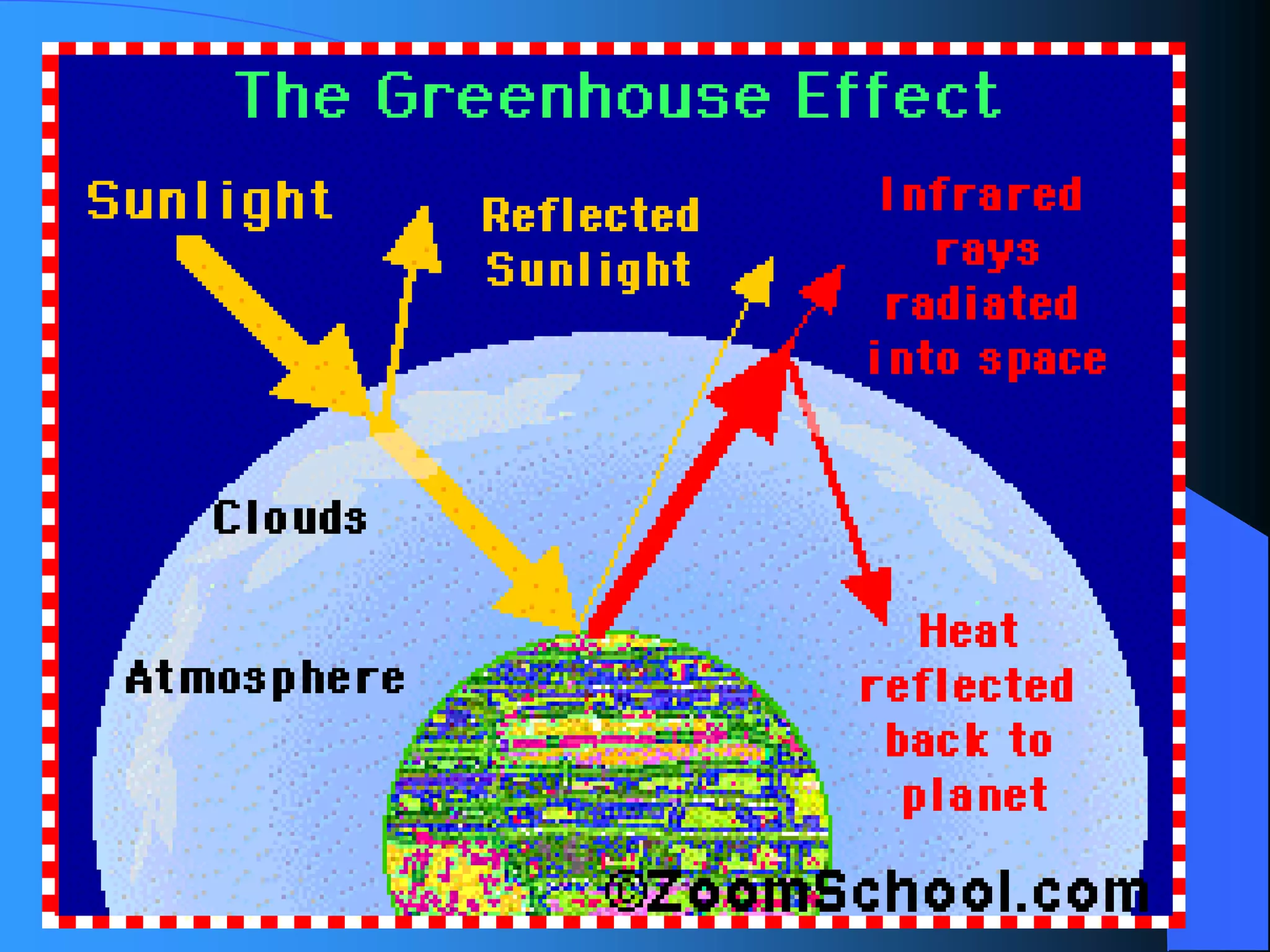
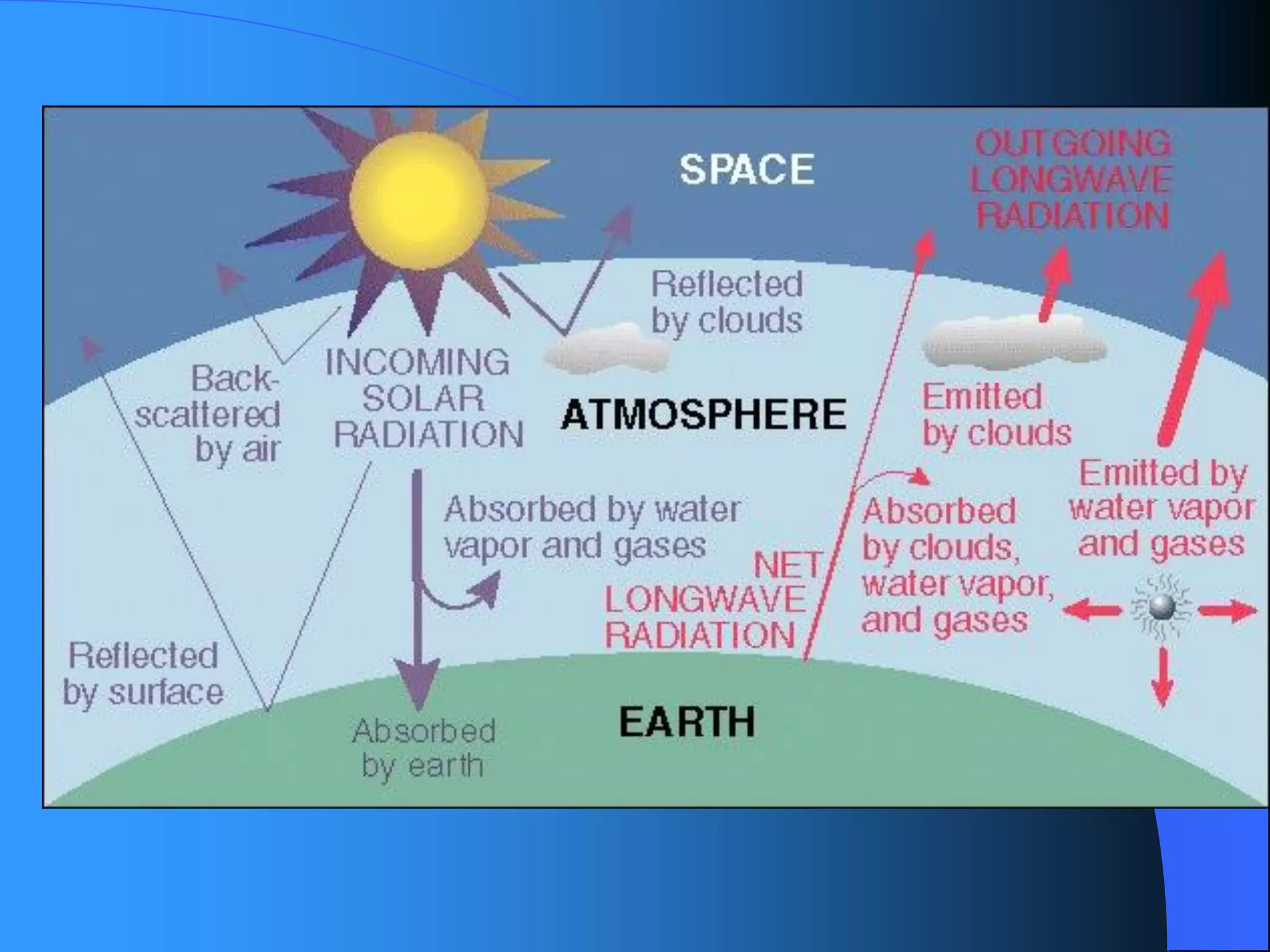
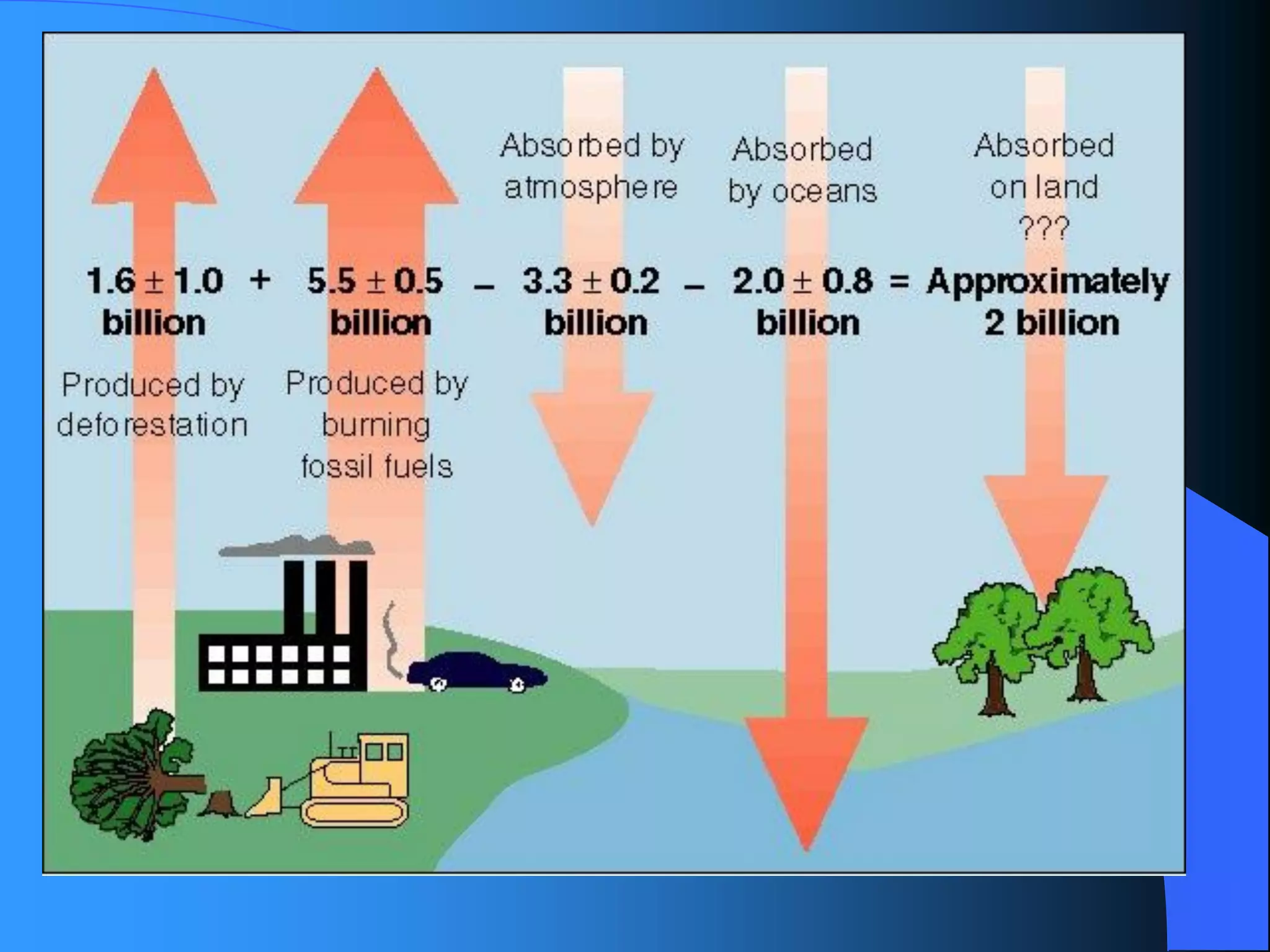
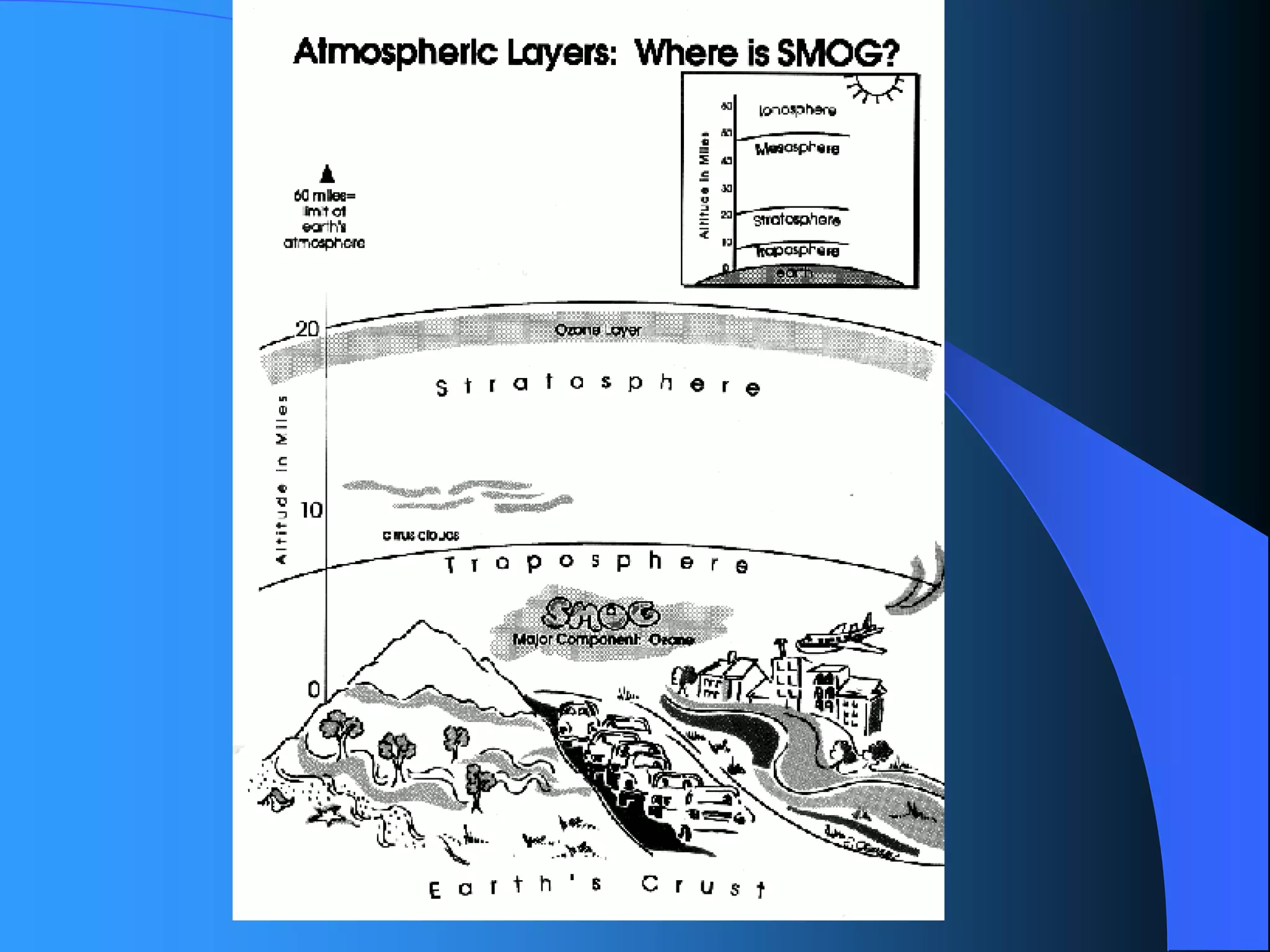
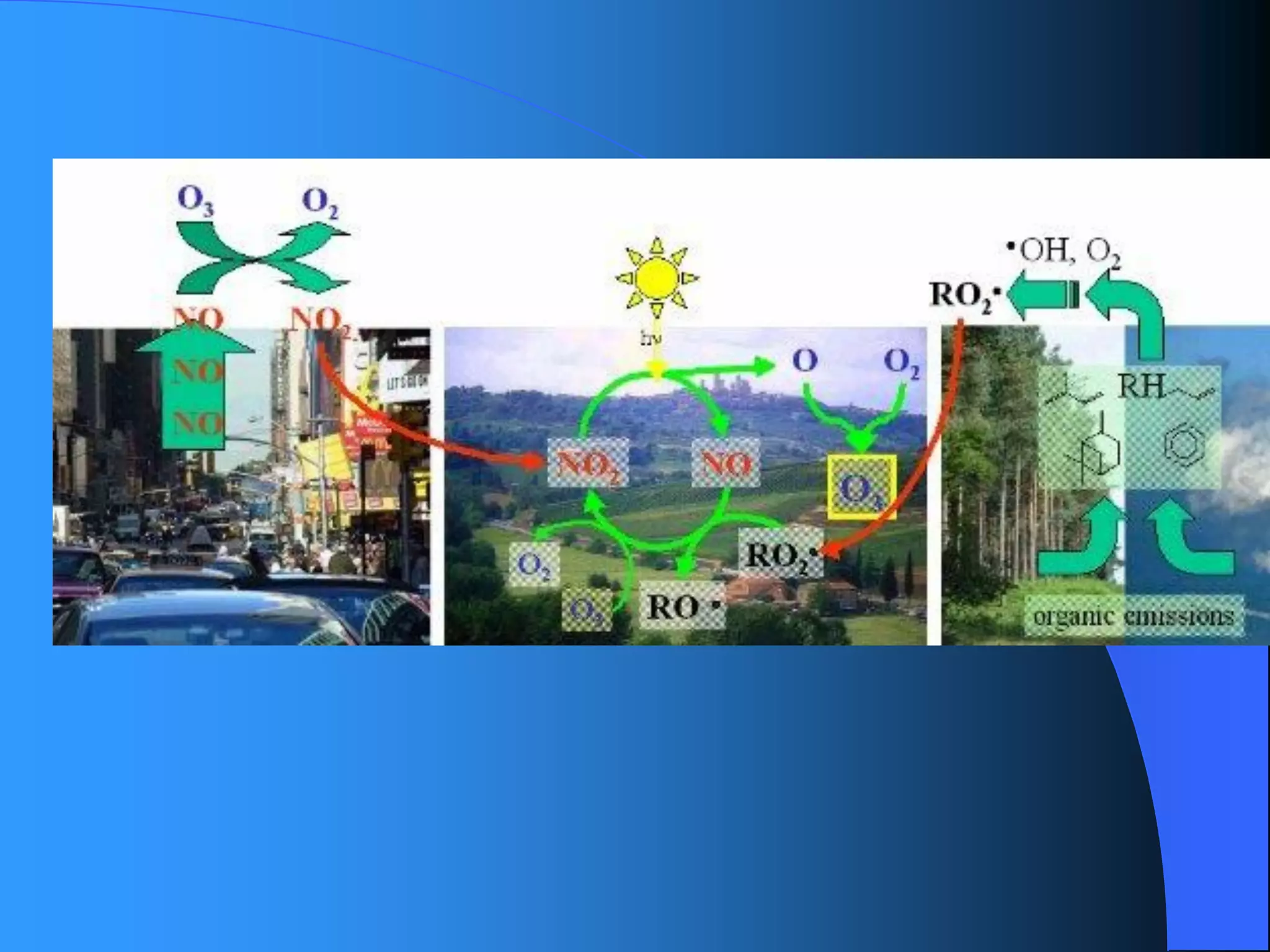
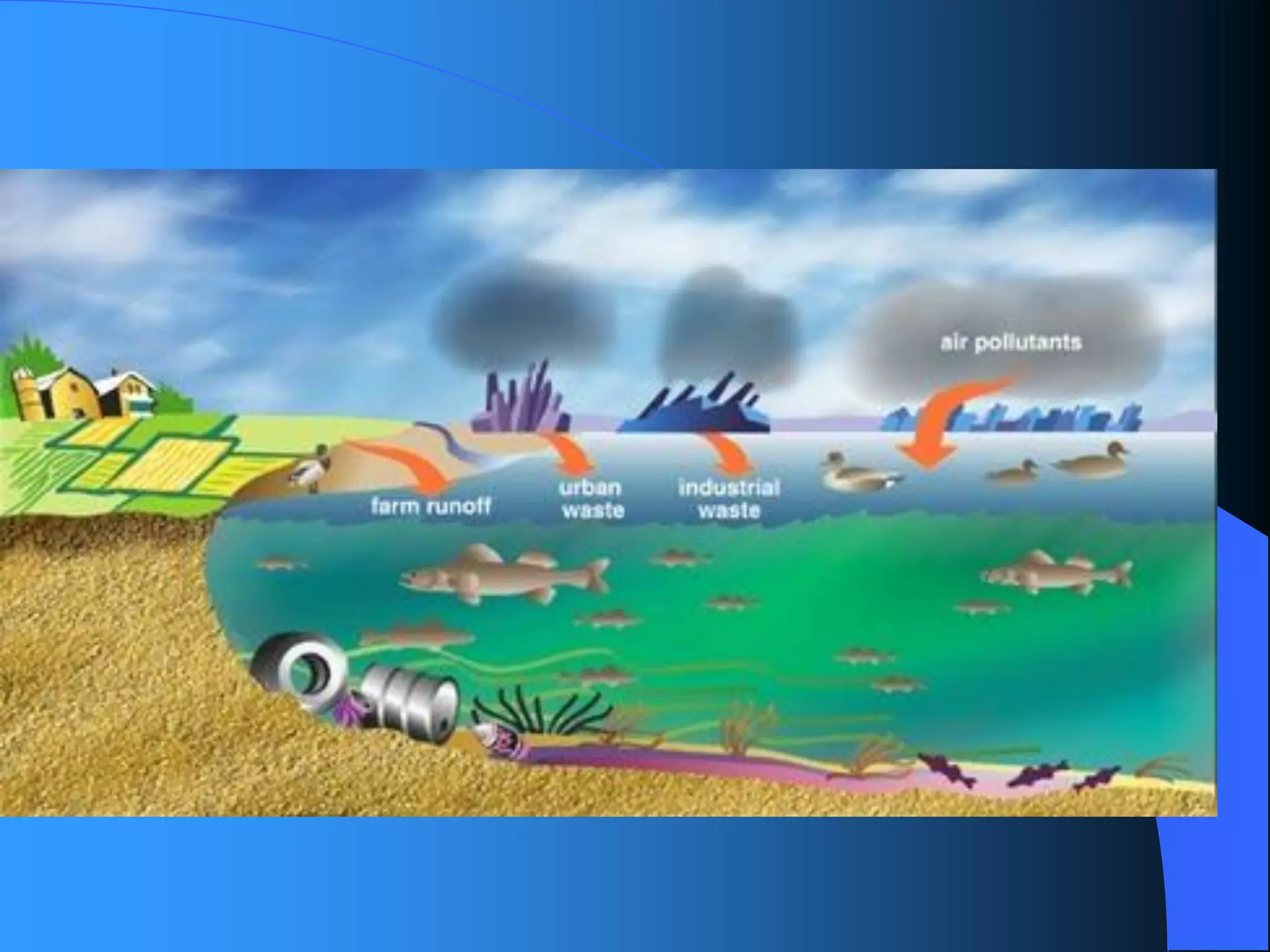
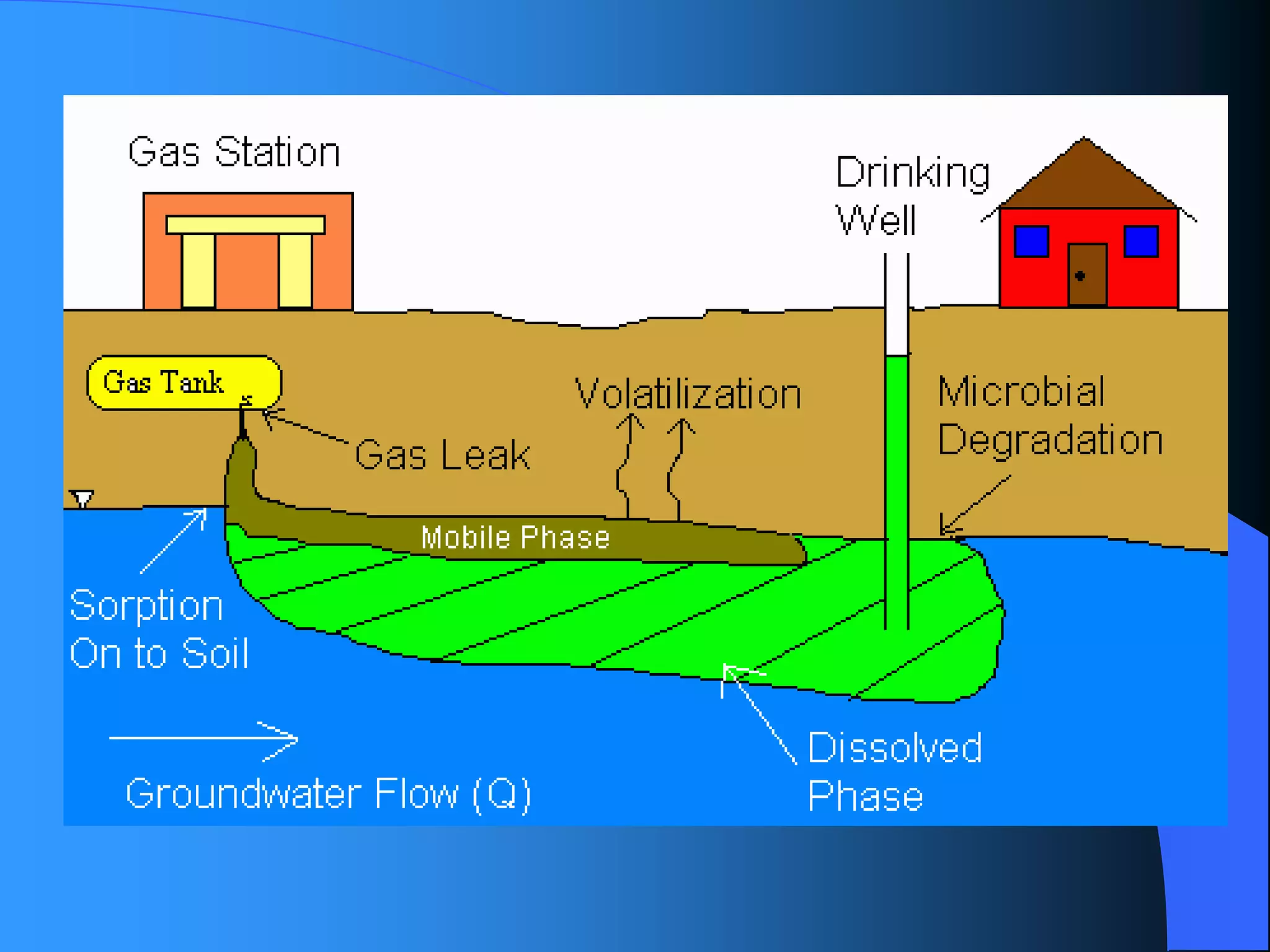
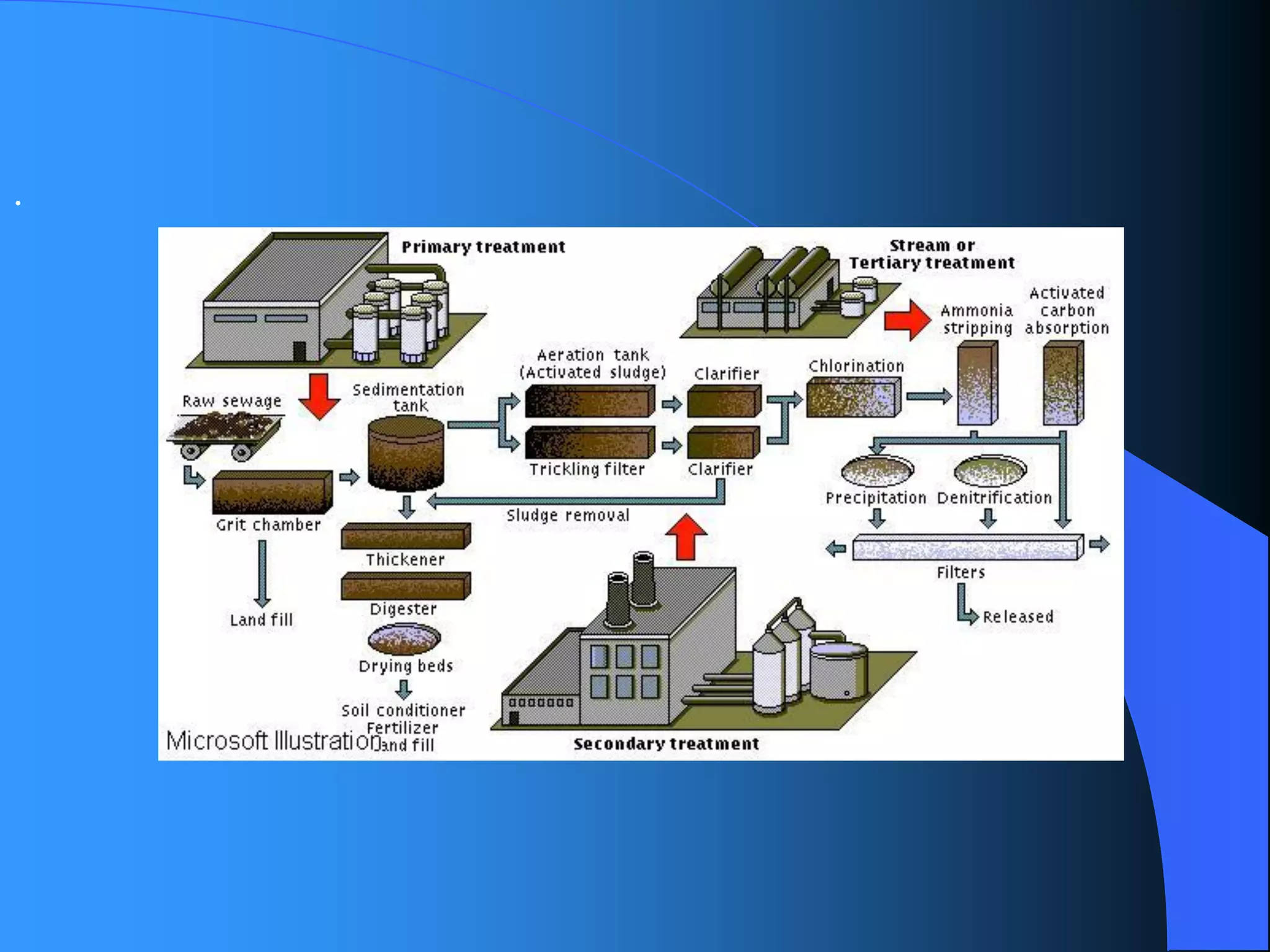
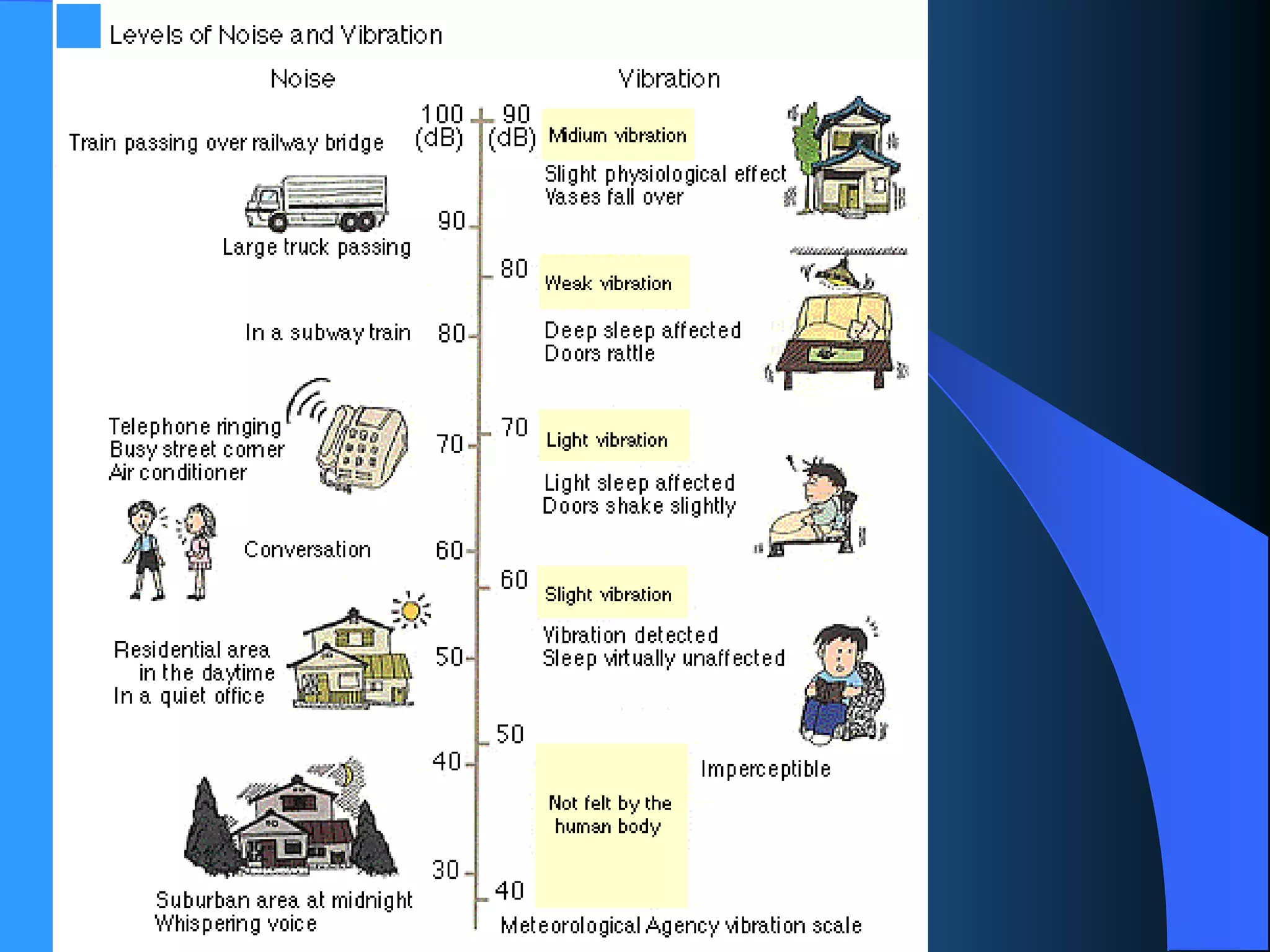
The document discusses environmental pollution, its causes, effects, and control measures, categorizing it into air, water, soil, noise, thermal, and nuclear pollution. It highlights the detrimental impacts of pollutants on health, ecosystems, and climate while emphasizing the importance of control measures and individual actions for prevention. The document serves as an overview of pollution types, sources, consequences, and the role of individuals in combating environmental degradation.