This document discusses a study that investigated the antifungal and anti-mycelium activities of biogenic silver, copper, zinc oxide, and gold nanoparticles. The nanoparticles were tested against four fungal strains (Candida albicans, Cryptococcus neoformans, Aspergillus niger, Fusarium oxysporum). The minimum inhibitory concentrations of silver, copper, and zinc oxide nanoparticles were determined to be ≤8 μg/ml for the non-spore forming fungi and ≤16 μg/ml for the spore forming fungi. Anti-mycelium effects were observed for A. niger and F. oxysporum, with silver nanoparticles showing the highest effect at 72.8%. The


![A. C. et al. / Environmental Nanotechnology, Monitoring & Management 5 (2016) 81–87 83
284. In addition, anti-mycelium activity of all four nanoparticles
was studied against A. niger MTCC 282 and F. oxysporum MTCC 284.
2. Materials and methods
2.1. Metal nanoparticles
Four different Biogenic nanoparticles; Silver (AgNPs) (Kelmani
et al., 2014), Copper (CuNPs) (Ashajyothi et al., 2014a,b), Zinc
oxide (ZnONPs) (Ashajyothi et al., 2014a,b) and Gold nanoparticles
(AuNPs) (Ashajyothi and Kelmani, 2014), were synthesized from
Enterococcus faecalis (Nonpathogenic) by extracellular method.
Organism was procured from Medical Biotechnology and Phage
Therapy Laboratory (MBPT), Department of Biotechnology, Gul-
barga University, Gulbarga.
2.2. Fungal strains
C. albicans MTCC 3017, C. neoformans MTCC 1347, A. niger MTCC
282 and F. oxysporum MTCC 284 (Microbial Type Culture Collec-
tion and Gene Bank, Chandigarh, India) were used to evaluate the
antifungal and anti-mycelium activities of biogenic nanoparticles.
C. albicans MTCC 3017 and C. neoformans MTCC 1347 were main-
tained on Sabouraud Maltose Agar (HiMedia, Mumbai, India), A.
niger MTCC 282 and F. oxysporum MTCC 284 on Potato Dextrose
Agar (HiMedia, Mumbai, India), and subculture at least twice on
the same medium at 35 ◦C for 48–72 h prior to use in experiments
to ensure optimal growth.
2.3. Assay for antifungal activity
The in vitro antifungal activity of the nanoparticles was eval-
uated using modified disk diffusion method. Sabouraud Maltose
Agar (for C. albicans MTCC 3017 and C. neoformans MTCC 1347) and
Potato Dextrose Agar (for A. niger MTCC 282 and F. oxysporum MTCC
284) was dispensed into separate petri dishes and allowed to solid-
ify. Spores were recovered by gentle swabbing the surface of the
culture plates using a sterile cotton swab; later the swab was dipped
in 5 ml sterile saline containing 0.1% Tween 80 to suspend the
spores. Serial dilution of spore suspension was performed to adjust
the initial inoculum to 4 × 104 spores/ml using counting cham-
ber. In aseptic conditions, 0.1 ml of spore suspension (adjusted to
4 × 104 spores/ml) was pipette onto the agar plates and suspen-
sion was spread uniformly. (Doughari and Nuya, 2008). Agar wells
of 5 mm diameter were made with the help of a sterilized stain-
less steel cork borer. Aseptic conditions were maintained during
the loading of different concentrations (20, 40, 60, 80, 100 g/ml)
of nanoparticle on marked agar wells using micropipette and water
was considered as control. Plates were incubated at 37 ◦C for 48 and
72 h. The zone of inhibition was measured in mm.
2.4. Assay for anti-mycelium activity
Anti-mycelium activity was performed using standard amended
nutrient agar method (Tomasino and Hamilton, 2006).Different
concentrations of each nanoparticle type samples were added
to autoclaved and cooled PDA and Sabouraud Maltose Agar. The
homogeneous mixture was poured in sterilized petri dishes. Seven
days old culture of A. niger MTCC 282 and F. oxysporum MTCC
284 was placed in the center of petri plate in two separate plates.
Three replicate plates were used per treatment. Plates containing
mycelium disc without nanoparticle and plates containing antifun-
gal drug (Amphotericin B) were considered as positive and negative
controls, respectively. All plates were incubated at 28 ± 2 ◦C for 4-
7 days. Fungal growth was measured as percent mycelia inhibition
by the formula:
Anti-mycelium activity(%) = [(Dc − Dt)/Dc] × 100
where, Dc: Diameter of colony in the control (mm), Dt: Diameter of
colony in the treatment (mm)
2.5. Determination of minimum inhibitory concentration
The MIC of nanoparticles was determined using the broth dilu-
tion method with slight modifications. Different concentrations (2,
4, 8, 16, 32, 64, and 128 g/ml) of nanoparticles were added to the
10 ml of potato dextrose broth with culture and incubated at 25 ◦C
for 48–72 h. The MICs were recorded after 48 h and the absorbance
was read at 600 nm.
3. Results and discussion
3.1. Nanoparticles used
In our published reports, FeSEM (Field emission Scanning elec-
tron Microscopy) and EDX (energy-dispersive X-ray) analysis were
used to determine the morphology, shape and chemical compo-
sition of biogenic nanoparticles. FeSEM images of functionalized
silver nanoparticles synthesized from E. faecalis bacterial biomass
can be seen with core shell morphology of size 9–130 nm and
marginal variation in the particle size was observed. Negligible
amount of phosphorus and sodium elements were showed by EDX
analysis (Kelmani et al., 2014). However copper nanoparticles, were
spherical in shape with size ranging from 20 to 90 nm. In addition,
EDX spectrum gives for copper nanoparticles showed two types of
signal peaks, one was for copper atom and another for elemental
oxygen (Ashajyothi et al., 2014a,b). Biogenic ZnO nanoparticles size
was ranging from 16 to 96 nm. The EDX spectrum reports showed
strong signals of Zinc atoms, along with signals from Oxygen, Potas-
sium, Phosphorus, Sulphate and Chloride atoms. (Ashajyothi et al.,
2014a,b). Similarly, FeSEM analysis of gold nanoparticles showed
the size range from 20 to 70 nm and EDX spectrum provided
number of signals from various contaminants, viz. sodium, chlo-
rine, potassium, calcium and oxygen elements (Ashajyothi et al.,
2014a,b).
3.2. Antifungal activity of nanoparticles against different
pathogenic fungi
The antifungal activity of biogenic nanoparticles against
pathogenic fungi was investigated using standard antifungal drugs
like Amphotericin B, Fluconazole, whereas AgNPs, CuNPs, ZnONPs
and AuNPs were used as comparable drugs. Inhibition effect of all
four biogenic nanoparticles at different concentrations were esti-
mated against C. albicans MTCC 3017 C. neoformans MTCC 1347, A.
Niger MTCC 282 and F. oxysporum MTCC 284 for 72 h of incubation
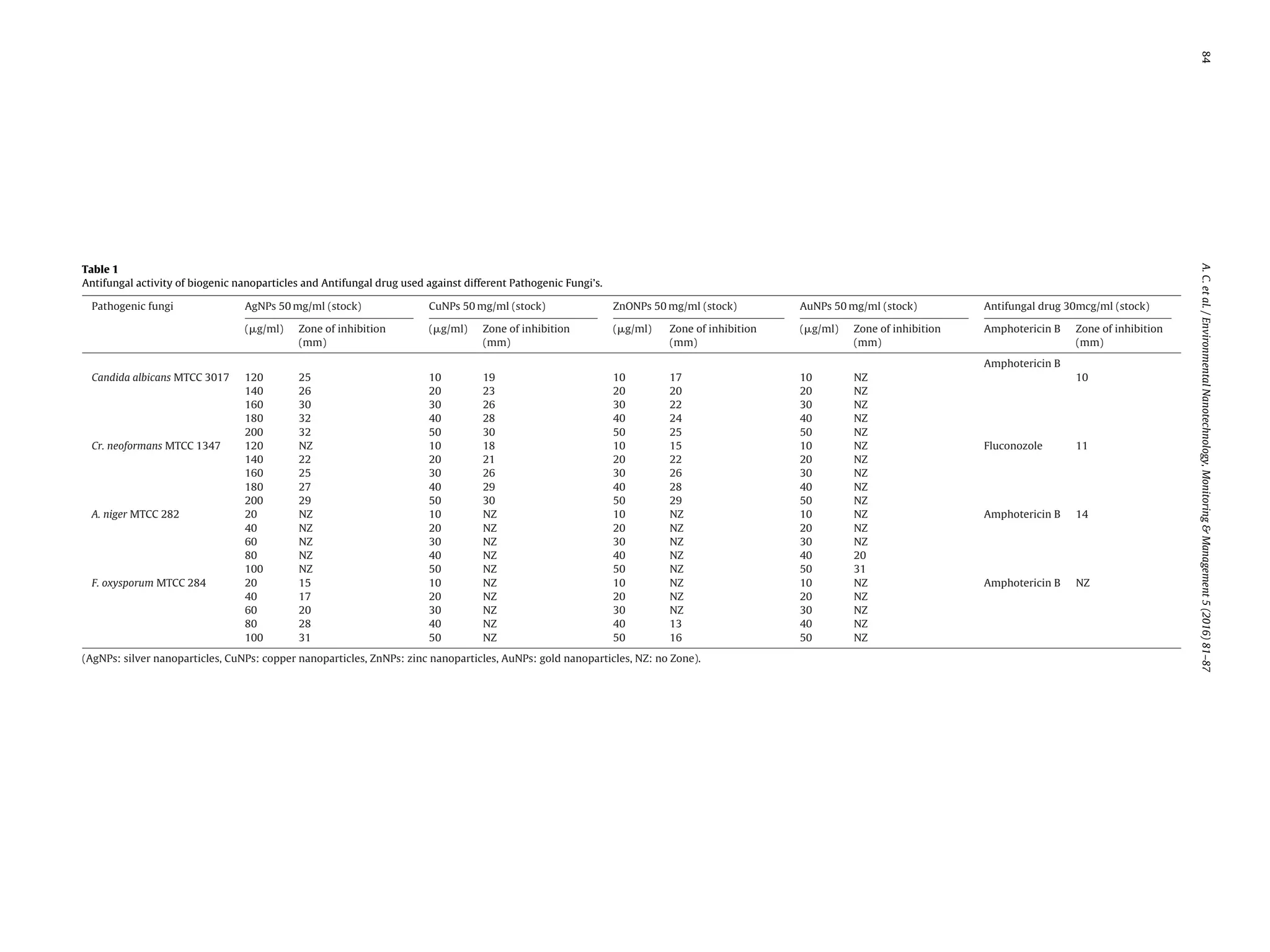
as represented in Table 1.
Silver nanoparticles treated with 200 g/ml concentration
showed highest fungal growth inhibition in C. albicans MTCC 3017
and C. neoformans MTCC 1347. According to Kim et al., 2007; spher-
ical shaped AgNPs were reported as potent drug against C. Albicans
when compared with commercially available antifungal drugs. In
microorganisms, Ag+ forms complexes with nucleotide base pairs
of DNA and also proven to be a potentially inhibit DNAases (Wen Ru
et al., 2010). AgNPs at 100 g/ml showed 31 mm of zone inhibition
against F. oxysporum MTCC 284 and failed to inhibit the growth of A.
niger MTCC 282. According to our studies, biogenic gold nanoparti-
cles showed less effectiveness towards all four pathogenic fungi’s,
except for A. niger MTCC 282.](https://image.slidesharecdn.com/9296d023-1537-4996-a745-a65d01cc8990-160919163048/75/ENMM-Paper-3-2048.jpg)