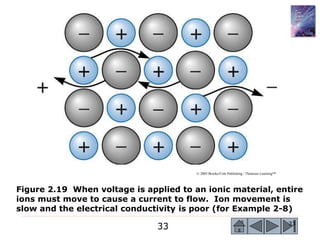
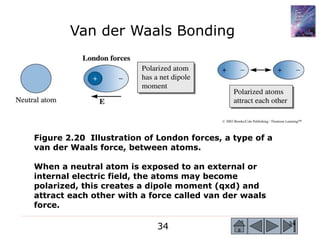
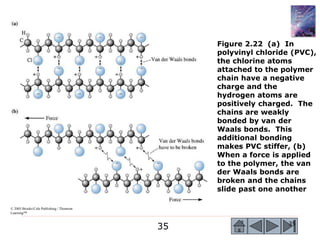
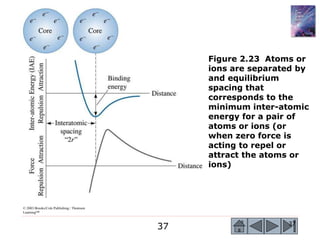
1) The document describes the structure of materials at different length scales from atomic to macroscopic levels. It discusses how atomic structure influences properties and technological applications of materials.
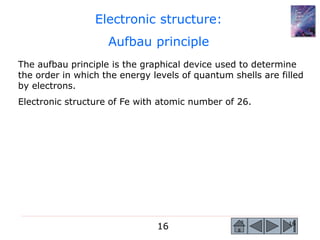
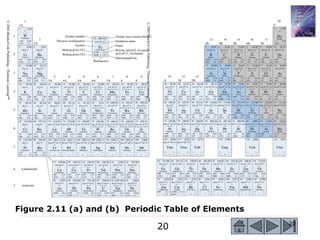
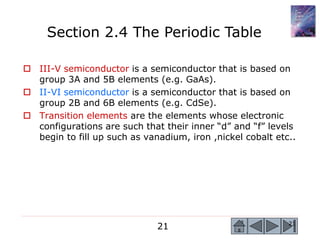
2) Key concepts covered include the structure of the atom, electronic configuration, periodic table, different types of atomic bonding, and how bonding influences properties like conductivity and strength.
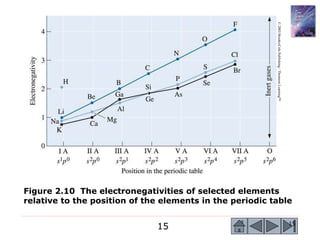
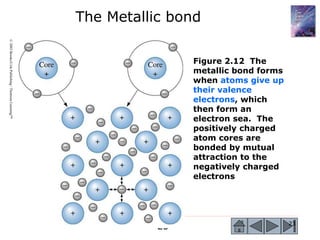
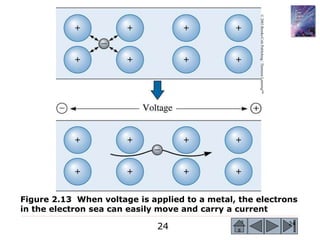
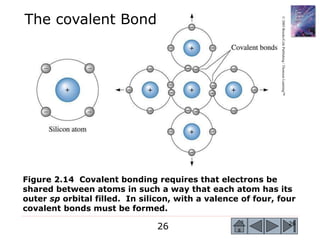
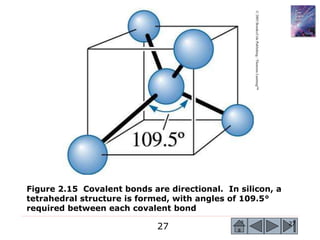
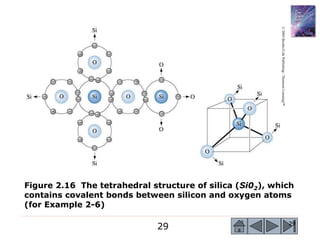
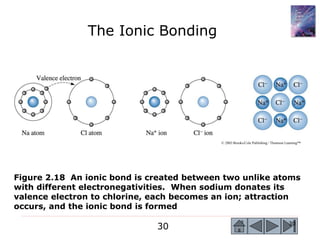
3) Examples calculate the number of atoms in materials and compare properties like electronegativity between elements. Diagrams illustrate different types of bonding and how structure determines properties.


![6 6
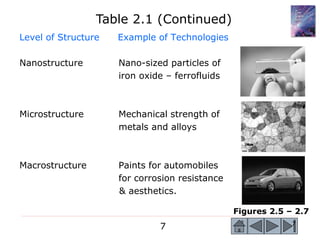
Level of Structure Example of Technologies
Atomic Structure Diamond – edge of
cutting tools
Atomic Arrangements: Lead-zirconium-titanate
Long-Range Order [Pb(Zrx Ti1-x )] or PZT –
(LRO) gas igniters, vib. control
Atomic Arrangements: Amorphous silica - fiber
Short-Range Order optical communications
(SRO) industry.
Figures 2.2 – 2.4
Table 2.1 Levels of Structure](https://image.slidesharecdn.com/lecture2-200621095140/85/engineering-material-atomic-structure-of-atom-Lecture2-6-320.jpg)