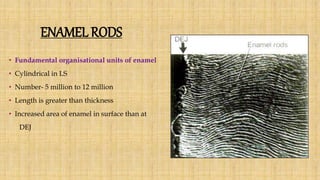
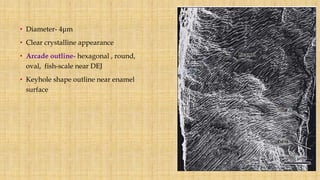
This document summarizes the structure and formation of enamel. It begins by describing enamel as the hardest substance in the body, composed primarily of hydroxyapatite crystals. It then discusses the composition, structure, and organization of enamel rods and crystals. Hunter-Schreger bands and incremental lines are described as well. The lifecycle of ameloblasts and stages of amelogenesis - morphogenic, organizing, formative, maturative, and protective - are summarized.

![INTRODUCTION
• Ectodermally derived structure produced by ameloblasts
• Hardest substance in the body
• Wear resistant outer layer of the dental crown
• Forms insulating barrier – protects the tooth
[Rodrigo.S.Lacruz et al: Dental enamel
formation and for oral health and disease,
May 2017]](https://image.slidesharecdn.com/enamel-170813162844/85/Enamel-structure-and-development-3-320.jpg)
![[Michel Goldberg el al : Dentin:
structure, composition and
mineralisation, January 2011]](https://image.slidesharecdn.com/enamel-170813162844/85/Enamel-structure-and-development-4-320.jpg)
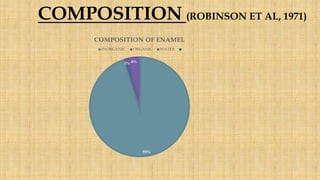
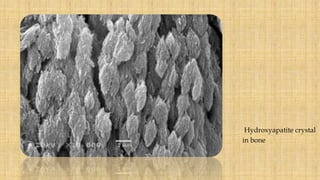
![• Inorganic component – mainly hydroxyapatite crystals, carbonates and trace
elements.
• Enamel hydroxyapatite crystals- largest of all calcified tissues
• Susceptible to dissolution of acid- basis of dental caries.
• Water is present as a part of hydroxyapatite crystal, boundaries of rods.
[ Jayasudha et al: Enamel regeneration- Current progress and challenges,
September 2014]](https://image.slidesharecdn.com/enamel-170813162844/85/Enamel-structure-and-development-7-320.jpg)

![• Carbonated calcium deficient hydroxyapatite-
tooth and bone
• Also seen in calcification within pineal gland -
Corpora arenacea (Brain sand)
• CO3 substitution for OH or PO4 – susceptible to
acidic dissolution- progression of caries
• F substitution – resistant to dissolution- caries
prevention and erosion reduction
[Rodrigo.S.Lacruz et al: Dental enamel formation
and implication for
oral health and disease, May 2017]](https://image.slidesharecdn.com/enamel-170813162844/85/Enamel-structure-and-development-10-320.jpg)


![Resistant covering Brittle
• Modulus of elasticity- 1338.2+307 MPa
• Hardness – 274.8+18.1 kg/mm
• Specific gravity- 2.8
[ K.J.Chun et al: Comparison of mechanical properties and role between enamel and dentin
in the human teeth, 2014]](https://image.slidesharecdn.com/enamel-170813162844/85/Enamel-structure-and-development-14-320.jpg)
![• Semipermeable membrane
- demonstrated by radioactive tracers and certain dyes
- (C- labelled urea, Iodine).
- The organic matrix and water in enamel is in a network of micropores-
dynamic connection between enamel and
systemic, pulpal or dentinal tubule fluids
- Micropores or cracks allow the penetration of fluids.
-Permeability decreases and hardness increases with age.
[Jansen et al: Permeability of normal enamel, 1951]
• Colour of enamel – yellowish white to greyish white
determined by translucency
[ K.J.Chun et al: Comparison of mechanical properties and role
between enamel and dentin in the human teeth, 2014]](https://image.slidesharecdn.com/enamel-170813162844/85/Enamel-structure-and-development-15-320.jpg)



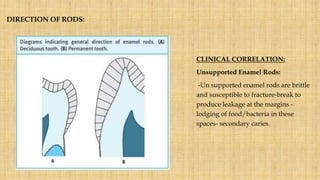

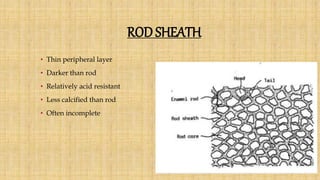
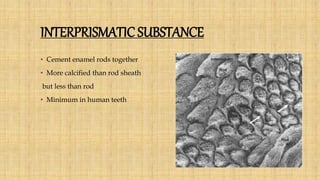




















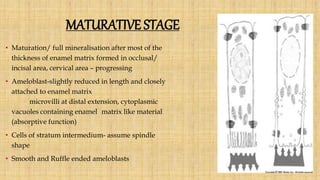
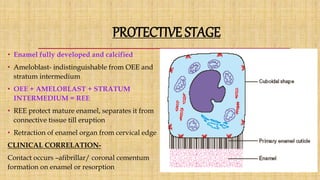
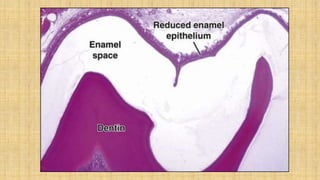







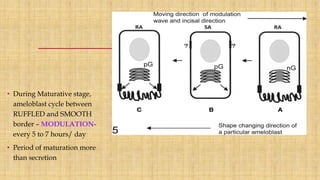

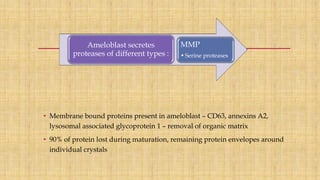




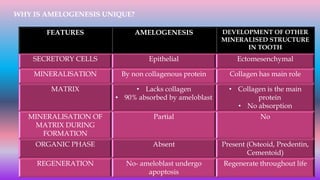

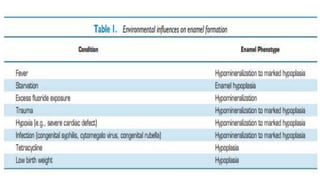

![Skin
epithelial
cells
Oral
keratinocytes
Human
embryonic
stem cell
derived
epithelial
cells
Bone marrow
cells
Epithelial cell
rests of
Malassez
Alternative cell sources for enamel
formation are-
[ Jayasudha et al: Enamel regeneration- Current
progress and challenges,
September 2014]](https://image.slidesharecdn.com/enamel-170813162844/85/Enamel-structure-and-development-93-320.jpg)

![CASEIN
CHITOSAN
GEL
EDTA
Example of some enamel biomimetic materials
[Rodrigo.S.Lacruz et al: Dental enamel formation
and implication for
oral health and disease, May 2017]](https://image.slidesharecdn.com/enamel-170813162844/85/Enamel-structure-and-development-95-320.jpg)


